Article Text
Abstract
Aims Because immunoglobulin abnormalities may affect the kidney, investigation of renal biopsies requires immunohistological study of light chains. A problem is that most antibodies to light chains react with whole immunoglobulins as well as free light chains, and there are generally many more whole immunoglobulins than free light chains. The usefulness of antibodies that only detected free light chains was investigated.
Methods Antibodies to free light chains were used in an immunoperoxidase method on paraffin sections of 198 renal biopsies, and compared with conventional antibodies against light chains examined by immunofluorescence on 13 frozen sections and by immunoperoxidase on 46 paraffin sections.
Results Immunofluorescence and immunoperoxidase were concordant on 10 of 13 biopsies. Immunofluorescence detected slight deposition of light chains in three biopsies not shown by immunoperoxidase, of undetermined clinical significance. Using immunoperoxidase, the free light chain antibodies were more sensitive than conventional antibodies, giving much cleaner staining and better detection of deposits in AL amyloid, light chain deposition disease and cryoglobulinaemic glomerulonephritis. The free light chain antibodies showed discordance or ambiguity between immunohistological and clinical findings in seven (4%) of 185 patients with known immunoglobulin status. These included two of 28 cases of AL amyloid that showed no light chain deposition. The method was not designed for detection of light chain restriction in neoplastic plasma or lymphoplasmacytic cells.
Conclusions Polyclonal antibodies to free light chains are an improvement on conventional antibodies in immunoperoxidase study of paraffin sections of renal biopsies and are useful in everyday practice.
- light chains
- sensitivity and specificity
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
Statistics from Altmetric.com
Introduction
Abnormal immunoglobulin light chains may have various effects on the kidney, especially cast nephropathy, AL amyloidosis or deposition disease, although they may have no detectable effects.1–4 Any study of light chains in renal biopsies, by immunofluorescence on frozen or paraffin sections or immunoperoxidase on paraffin sections, has disadvantages, including the specially collected material required for frozen sections, impermanence of fluorescence, and pretreatment usually needed for paraffin sections, but most antibodies give comparable findings between frozen and paraffin sections.5–9
A problem is the specificity of antibodies against light chains. Most polyclonal antibodies react with light chains both free and bound to heavy chains, but usually react preferentially with whole immunoglobulins, because normal mean plasma concentrations of free light chains and whole immunoglobulins are about 23 mg/L10 and 19 g/L,11 one thousand times different. Monoclonal antibodies may be specific for a free light chain, but the epitope recognised may not be expressed or accessible in all light chains.12
Polyclonal antibodies specific for free light chains are available,10 with applications in measurement of plasma light chains and diagnosis and management of clinical immunoglobulin disorders.13 Our study assessed the value of these antibodies in immunohistology of paraffin sections of renal biopsies, including comparison with conventional antibodies.
Materials and methods
Antibodies
Sheep antibodies to human free κ and λ light chains were supplied by The Binding Site, Birmingham, UK. Rabbit antibodies to κ and λ (A0191, A0193), mouse antibody to serum amyloid A (M0759) and fluorescein-conjugated antibodies to κ and λ (F0198, F0199) were obtained from Dako.
Immunostaining
For immunoperoxidase, endogenous peroxidase was blocked on dewaxed 3 µm paraffin sections by hydrogen peroxide. After preliminary experiments, optimum antigen retrieval for sheep antibodies was digestion with protease type 24 (Sigma, P8038), 0.05% w/v in phosphate buffered saline pH 7.2, at 37°C, initially for 45 min, with microscopic study of completeness of digestion and further digestion if necessary.5 Antibodies were applied at 1:400 (κ) or 1:200 (λ) for 45 min, then rabbit anti-sheep immunoglobulins, peroxidase-conjugated (Dako, P0163), at 1:100, for 45 min, followed by diaminobenzidine and hydrogen peroxide, and haematoxylin. Various methods of antigen retrieval for rabbit antibodies were tried, including no pretreatment, protease digestion, trypsin digestion, microwave heating, pressure cooking, and heating for 30 min in antigen retrieval solution pH 9.0 provided in a kit for an automated method (Bondmax: Leica Microsystems, Milton Keynes, UK). Most pretreatments matched automated antigen retrieval, but protease abolished immunostaining and no pretreatment gave weak immunostaining. Antibodies were applied at 1:20 000, followed by Bondmax anti-rabbit Poly-HRP-IgG, peroxidase substrate and counterstain. Amyloid A antibody (1:500) was used without pretreatment followed by Bondmax anti-mouse antibody. Frozen sections were covered with fluoresceinated antibodies and examined by fluorescence microscopy.
Thirty-two patients were referred to the National Amyloidosis Centre (NAC). Their renal biopsies were independently examined with the rabbit antibodies to light chains without antigen retrieval, and with antibodies to amyloid A and if necessary to other potential amyloid precursors.14
Renal biopsies
Thirteen biopsies examined by immunofluorescence on frozen sections were studied using the free light chain antibodies on paraffin sections, as were another 185 paraffin-embedded biopsies. These included 46 biopsies used to compare the free light chain and rabbit antibodies, 32 at NAC and 14 others. Immunostained paraffin sections were examined without knowledge of clinical features or original reports of light chain findings. Diagnoses were made in usual ways.15 Conventional summary statistics (sensitivity, specificity and CIs of differences in proportions) were calculated.
Evidence of immunoglobulin disorders
Apart from three cases studied by immunofluorescence, all others had evidence for or against a disorder of immunoglobulins. Evidence was any of these: a serum paraprotein; a cryoglobulin; an abnormal ratio of serum free κ to λ concentrations10; or a monoclonal (Bence Jones) light chain in urine. The Results mostly concentrate on the 185 biopsies, selected because there was adequate information about immunoglobulin status of the patients, 110 with an immunoglobulin abnormality and 75 without. Free light chain immunostaining was more likely to be done if an abnormality was known or suspected or was in the differential diagnosis.
Results
Comparison of immunofluorescence and immunoperoxidase
Ten of 13 biopsies showed agreement between the methods, with AL amyloid (5), no light chain deposition (3), and deposition disease (2). The other three showed no free light chain deposition, and only faint deposition on immunofluorescence, one of κ in tubular basement membranes with slight chronic damage, one of κ in glomerular basement membranes but no structural glomerular abnormality, and one of more λ than κ in tubular basement membranes but no clinical immunoglobulin abnormality.
Comparison of different antibodies using immunoperoxidase
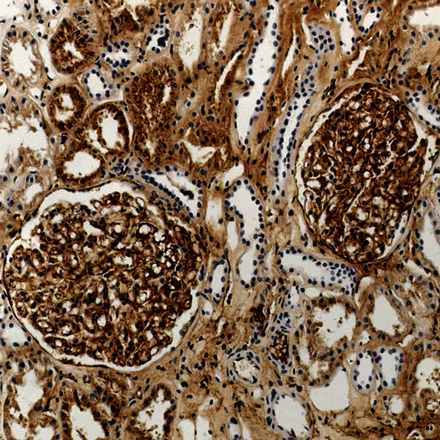
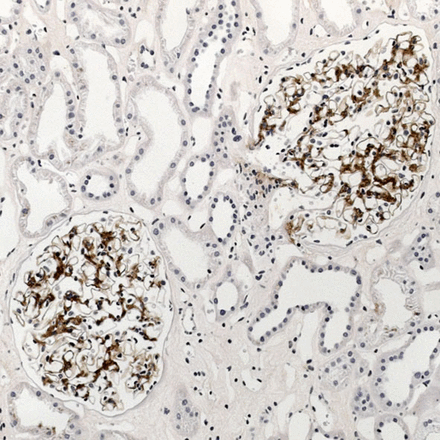
The 14 non-NAC biopsies comparing free light chain and rabbit antibodies had cast nephropathy (3), amyloid (3, two AL, one AA), no immunoglobulin abnormality (3), renal infiltration by lymphoplasmacytic lymphoma or myeloma (2), cryoglobulinaemic glomerulonephritis (2, both type II) and deposition disease (1). Rabbit antibodies after automated antigen retrieval showed heavy staining of tubular epithelium, interstitial tissues and blood, but background staining was much lighter without pretreatment (figures 1, 2). After automated antigen retrieval, deposits of light chains in AL amyloid, cryoglobulinaemia and deposition disease were impossible to distinguish from the background, and without pretreatment, immunostaining for light chains was weak and only seen in one case, with AL amyloid. Deposits were easily detected with free light chain antibodies, with clean background and no blood in vessels (figures 3, 4).
Renal biopsy containing amyloid in glomeruli in a patient with a κ paraprotein. Immunostaining with the rabbit antibody to κ light chains after automated antigen retrieval shows extensive staining but no detectable selective staining of amyloid deposits.
The same renal biopsy as in figure 1. Immunostaining with the rabbit antibody to κ light chains without pretreatment shows light background staining but no detectable staining of amyloid deposits.
The same renal biopsy as in figure 1. Immunostaining with the sheep antibody to free κ light chains shows selective staining of amyloid deposits in glomeruli.
The same renal biopsy as in figure 1. Immunostaining with the sheep antibody to free λ light chains shows no staining.
Both types of antibody showed a preponderance of one light chain in casts and tubular epithelium in cast nephropathy. Light chain restriction was identified in the myelomatous infiltrate only with rabbit antibodies, but with neither antibody in the lymphoplasmacytic lymphoma. Comparison of the antibodies, including findings in NAC (below), is in table 1.
Comparison of diagnostic accuracy of antibodies to free light chains and conventional antibodies to light chains in 46 renal biopsies, compared with final diagnosis of any renal abnormality related to light chain disorders, including neoplastic infiltration
Amyloid
With free light chain antibodies, 25 of 41 biopsies showed AL amyloid (21 λ, four κ), with concordance between clinical and immunoperoxidase findings (tables 2, 3). NAC confirmed λ AL amyloid in 13 of 17 λ cases and κ AL amyloid in one of two κ cases by immunohistology, and in four and one, respectively, from overall evidence.
Light chain immunohistological findings in 41 renal biopsies containing amyloid
Summary of immunohistological findings with antibodies to free light chains in 185 renal biopsies and immunoglobulin status of patients (as defined in materials and methods)
Eight biopsies showed AA amyloid and three nonAL, nonAA amyloid with no free light chain immunostaining, concordant with no immunoglobulin disorder. NAC confirmed five cases of AA amyloid by immunohistology, and identified two nonAL, nonAA cases as LECT2 amyloid and one as fibrinogen A α amyloid. Another case had no clinical immunoglobulin abnormality, but amyloid deposits reacted with antibodies to amyloid A and free κ. NAC diagnosed AA amyloid immunohistologically.
The other four cases had a clinical immunoglobulin abnormality. One with a κ paraprotein showed deposition of both free κ and amyloid A. NAC showed no deposition on immunohistology but considered this κ AL amyloid on overall evidence. In the other three, no free light chain deposition was detected. One with a λ Bence Jones protein was confirmed λ AL amyloid immunohistologically at NAC. One with a λ paraprotein had no immunohistological deposition at NAC but was considered λ AL amyloid on overall evidence. In one case with a κ paraprotein, the amyloid reacted only with amyloid A antibody. AA amyloid was confirmed by NAC.
Other conditions
Light chain cast nephropathy
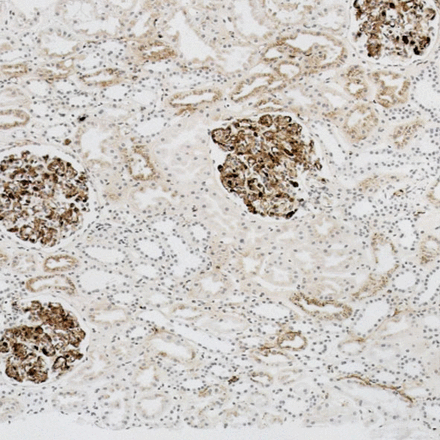
Findings were concordant between immunostaining for free light chains and immunoglobulin abnormalities in 32 of 33 cases (26 κ, six λ), with definite preponderance of one light chain in casts and tubular epithelial cells (figures 5, 6). In the discordant case, there was a κ paraprotein and a few casts showed anomalous colours with Congo red as found in some cases of cast nephropathy, without definite preponderance of κ on immunostaining.
Renal biopsy showing light chain cast nephropathy in a patient with an IgA λ paraprotein. Immunostaining with the sheep antibody to free λ light chains shows deposition in casts.
Light chain deposition disease
Findings were concordant in all six cases of deposition disease (five κ, one λ), with glomerular deposition of the appropriate light chain, and often deposition in tubular basement membranes (figure 7).
Renal biopsy showing light chain deposition disease in a patient with a κ paraprotein. Immunostaining with the sheep antibody to free κ light chains shows deposition in mesangium and tubular basement membranes. There was no deposition of free λ light chains.
Other renal effects of immunoglobulin abnormalities
In four of 11 cases, free light chain immunostaining did not show a preponderance of one light chain. Two were kidneys infiltrated by myeloma and lymphoplasmacytic lymphoma, and two were cases of cryoglobulinaemia (one type II, one type III). There was concordance in the other seven cases, all κ, four cryoglobulinaemic glomerulonephritis (one type I, three type II) (figure 8), two tubulointerstitial nephritis with light chains in tubular basement membranes, and one with crystalline deposits of light chains in podocytes.
Renal biopsy showing type II cryoglobulinaemic glomerulonephritis in a patient with an IgM κ paraprotein. Immunostaining with the sheep antibody to free κ light chains shows extensive deposition in glomeruli. There was no deposition of free λ light chains.
No evidence of renal effects of an immunoglobulin abnormality
In 31 cases, there was no detectable effect of a paraprotein in the kidney and no immunohistological disproportion between κ and λ. The commonest diagnoses were diabetic glomerulopathy (6), membranous nephropathy (4) and chronic ischaemic damage (4).
No immunoglobulin abnormality
Excluding 12 amyloid cases, 63 biopsies were from patients without an immunoglobulin abnormality as defined above, although many had immunoglobulin deposits, shown by antibodies to heavy chains. In 62 there was no disproportion between κ and λ on immunohistology. One showed subendothelial membranoproliferative glomerulonephritis with more κ than λ in glomerular deposits, without evidence of a monoclonal gammopathy.
Discussion
Immunofluorescence on frozen sections and immunoperoxidase or immunofluorescence on paraffin sections rarely show complete concordance in detection of light chain deposition. Which method gives the correct or more clinically relevant finding is undetermined.6–9 The discrepancies in our study were minor and of uncertain clinical significance.
Comparison on paraffin sections between free light chain and conventional antibodies showed that differences were due to the antibodies, not pretreatments. The free light chain antibodies did not detect whole immunoglobulins and were more sensitive than conventional antibodies (table 1). Light chains in deposits were readily differentiated from background. The free light chain antibodies were better at typing AL amyloid, giving stronger and generally unequivocal staining (figures 3, 4; table 2). Two of 28 cases (7%) of AL amyloid were missed, although to different extents all antibodies to light chains miss cases of AL amyloid, up to 35% in one series.14 ,16 ,17 Some cases of AL amyloid will still need other investigations for definitive diagnosis, such as laser microdissection of sections and mass spectrometry.17 ,18 Reactivity of amyloid with multiple antibodies has been reported, for instance in 34% of biopsies with AA amyloid,19 and two of our 41 amyloid cases (5%) showed ambiguous immunostaining for both free κ and amyloid A. Four amyloid cases (10%) therefore showed discordance between clinical and immunohistological findings, overlooking one case with a paraprotein but confirmed to be AA amyloid.
The free light chain antibodies were useful in confirmation of deposition disease, with complete concordance, and usually of cryoglobulinaemic glomerulonephritis (figures 7, 8). The discordant case of type II cryoglobulinaemia, with mixed monoclonal IgM and polyclonal IgG, may have had insufficient excess of free light chains to be detectable. In type III cryoglobulinaemia, with polyclonal deposits, no excess of one free light chain would be expected, and was not considered discordant. There was little difference between free light chain and conventional antibodies in detection of light chains in cast nephropathy. The failure of free light chain antibodies to show an excess of one light chain in one of 33 cases (3%) may be because in virtually every case of cast nephropathy both light chains are found (figures 5, 6), and a small excess of one may be undetectable.
The free light chain method was developed to detect deposits rather than intracellular or surface immunoglobulins, and neoplastic infiltrates can be investigated in other ways.20 There was no deposition of free light chains in 32 cases with a monoclonal gammopathy but without diagnosable renal effects, including one case of AA amyloid (29% of those with immunoglobulin abnormalities). Similarly, 27% of patients with myeloma3 and 63% with a monoclonal gammopathy1 had no evidence of renal lesions related to a paraprotein. Accordingly, only seven of 185 cases (4%) were considered discordant: four amyloid, one cryoglobulinaemia, one light chain cast nephropathy, and one glomerulonephritis with excess κ deposition but no gammopathy.
Polyclonal free light chain antibodies are of practical use in the study of renal biopsies and have advantages over conventional light chain antibodies. Methods are similar and overall costs are also likely to be similar.
Take home messages
-
Polyclonal antibodies to free light chains are an improvement on conventional antibodies to light chains in the immunoperoxidase study of paraffin sections of renal biopsy specimens, giving a cleaner background and sharper discrimination of sites of deposition.
-
These antibodies are more sensitive than conventional antibodies in the detection of cases of immunological disorders in the kidney such as AL amyloid, light chain deposition disease and cryoglobulinaemic glomerulonephritis.
Acknowledgments
We are grateful to The Binding Site Group Ltd (UK) (http://www.thebindingsite.com/) for the antibodies to free light chains.
References
Footnotes
-
Contributors MPOC and AJH planned the study, analysed the immunoperoxidase findings and wrote initial drafts of the paper. RS developed and performed the free light chain immunostaining. HTC and CAR analysed the immunofluorescence findings. JDG and JAG performed and analysed the immunostaining at NAC. CAH collaborated with The Binding Site Group Ltd (UK) to obtain the antibodies to free light chains. All authors contributed to revisions of the manuscript and approved the final version.
-
Competing interests CAH reports grants and personal fees from The Binding Site Group Ltd (UK) outside the submitted work.
-
Ethics approval NRES Committee London (Hampstead).
-
Provenance and peer review Not commissioned; externally peer reviewed.