Article Text
Statistics from Altmetric.com
Introduction
Oral cancers are the 10th most common cancer worldwide, with oral tongue squamous cell carcinoma (OTSCC) having the highest incidence.1 Surgery and radiotherapy (RT), the mainstay treatments for OTSCC, impact on the patients' quality of life and the 50% 5-year survival has remained unchanged for 40 years.1
Cancer stem cells (CSCs) have been proposed to be the origin of many cancers, including oral cavity squamous cell carcinoma (OCSCC). CSCs express the embryonic stem cell (ESC) markers OCT4,2 NANOG,3 SOX2,2 SALL43 and STAT3,4 the more ‘downstream’ CSC marker CD442 and the epithelial cell marker p63,5 suggesting a diverse phenotype. The relative abundance and co-expression of these markers and their localisation within OCSCC remain unclear.
Immunohistochemistry
4 μm thick formalin-fixed paraffin-embedded sections of moderately differentiated OTSCC (MDOTSCC) samples from seven male and three female patients, aged 30–84 (mean 63.3) (see online supplementary table S1) were used for immunohistochemical (IHC) staining, as previously described.6 All sections underwent single 3,3-diaminobenzidine (DAB) IHC staining for the primary antibodies NANOG, 1:500 (cat#D73G4; Cell Signaling Technology, Danvers, Massachusetts, USA), SOX2, 1:500 (cat#PA1-094; Thermo Fisher Scientific, Santa Cruz, California, USA), CD44, 1:1500 (cat#MRQ-13; Cell Marque, Santa Cruz, California, USA), pSTAT-3, 1:100 (cat#D3A7; Cell Signaling Technology), SALL4, 1:100 (cat#6E3; Cell Marque) and OCT4, 1:30 (cat#MRQ-10; Cell Marque), Bond Ready-To-Use p63 (cat#NCL-p63; Leica, Newcastle, UK). To confirm co-expression of two proteins, representative slides of MDOTSCC (n=2) underwent immunofluorescent (IF) IHC staining using a combination of VectaFluor Excel anti-rabbit 594 (ready-to-use, cat#VEDK-1594, Vector Laboratories, Burlingame, CA, USA) and Alexa Fluor anti-mouse 488 (1:500, cat#A21202, Life Technologies) to detect combinations that included NANOG, SOX2 and pSTAT3 and VectaFluor Excel anti-mouse (ready-to-use, cat#VEDK2488, Vector Laboratories) and Alexa Fluor anti-rabbit 594 (1:500, cat#A21207, Life Technologies) to detect combinations that included OCT4 or SALL4. Positive control tissues used were human skin (for p63), tonsil (for CD44) and seminoma (for OCT4, NANOG, SOX2, SALL4 and pSTAT3).
Supplementary table
DAB IHC staining of MDOTSCC samples revealed expression of p63 (see online supplementary figure S1A, brown), SALL4 (see online supplementary figure S1B, brown), SOX2 (see online supplementary figure S1C, brown), NANOG (see online supplementary figure S1D, brown), pSTAT3 (see online supplementary figure S1E, brown) and CD44 (see online supplementary figure S1F, brown) by cells predominantly localised to the tumour nests (TNs), distinct from the cells expressing OCT4 (see online supplementary figure S1G, brown) within the stroma, between the TNs.
Supplementary figure
IF IHC staining demonstrated nuclear expression of p63 in cells within the TNs (figure 1A, green). SOX2 (figure 1A, red) was expressed by the p63+ (figure 1A, green) immunoreactive (IR) cells. OCT4 (figure 1B, green) and SOX2 (figure 1B, red) were expressed by two distinct population of cells. SALL4+ (figure 1D, G, green) cells expressed NANOG (figure 1D, red) and SOX2 (figure 1E, red). This SOX2+ (figure 1F, red) population also demonstrated membranous expression of CD44 (figure 1F, green) and was also IR for pSTAT3 (figure 1G, red). Specificity of the primary antibodies was confirmed with the positive controls (data not shown).
Representation of immunofluorescent (IF) immunohistochemical (IHC)-stained sections of 10 moderately differentiated oral tongue squamous cell carcinoma, demonstrating nuclear co-expression of the epithelial cell marker p63 (A, green) and the cancer stem cell (CSC) marker SOX2 (A, red), within the tumour nests. The SOX2+ population (B, red) was distinct from the CSC population that expressed OCT4 (B, green). The OCT4+ (C, green) cells were distinct from the CSC that expressed NANOG (C, red). Further staining with SALL4 demonstrated that the SALL4+ cells (D and E, green) also expressed NANOG (D, red) and SOX2 (E, red); this combination of the green and red stains displayed as orange (D and E). The SOX2+ (F, red) cells also expressed the membranous CSC marker CD44 (F, green). The CD44+ (G, green) cells also expressed the CSC marker pSTAT3 (G, red). Scale bars: 20 μm.
Nanostring mRNA analysis
Total RNA, isolated and quantified as previously described6 from six snap-frozen MDOTSCC samples of the original cohort of 10 patients used for DAB IHC staining, was subjected to the NanoString nCounter gene expression assay (NanoString Technologies, Seattle, WA, USA) performed by New Zealand Genomics (Dunedin, New Zealand). Probes for the genes encoding P63 (NM_003722.4), CD44 (NM_001001392.1), OCT4 (NM_002701.4), NANOG (NM_024865.2) and STAT3 (NM_139276.2) and the housekeeping genes GUSB (NM_00181.3), CLTC (NM_4859.2) and HPRT1 (NM_000194.1) were used. Data were analysed by nSolver software (Nanostring Technologies).
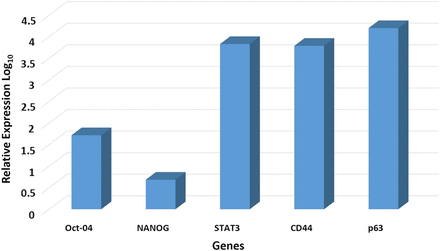
NanoString transcriptional profiling of six MDOTSCC samples, normalised against the housekeeping genes, glucuronidase beta (GUSB), clathrin heavy chain (CLTC) and HPRT1, confirmed the presence of mRNA for p63, epithelial membrane antigen (EMA), CD44, STAT3, OCT4 and NANOG (figure 2).
Copy number variants for mRNA transcripts in six moderately differentiated oral tongue squamous cell carcinoma for OCT4, SOX2, NANOG, STAT3 and p63 expressed relative to the housekeeping genes GUSB, CLTC and HPRT1.
In situ hybridisation
Representative 4 μm thick formalin-fixed paraffin-embedded sections of three MDOTSCC samples were used for mRNA in situ hybridisation (ISH) staining, as previously described.6 The probes used for OCT4 (NM_002701.4), NANOG (NM_024865.2), SOX2 (NM_003106), STAT3 (NM_139276.2) and SALL4 (NM_020436) were obtained from Affymetrix (CA, USA). Human seminoma tissue sections were used as positive controls.
ISH staining localised mRNA for OCT4 (figure 3A, red), SOX2 (figure 3B, red), STAT3 (figure 3C, red), NANOG (figure 3D, red) and SALL4 (figure 3E, red) to the nuclei of OTSCC epithelial-like cells. Specificity of the ISH probes was confirmed with positive staining in human seminoma (data not shown).
Representative in situ hybridisation-stained formalin-fixed paraffin-embedded oral tongue squamous cell carcinoma sections demonstrating mRNA expression of OCT4 (A, red, arrows), SOX2 (B, red, arrows), STAT3 (C, red, arrows), NANOG (D, red, arrows) and SALL4 (E, red, arrows). Original magnification: ×1000.
Imaging
All DAB IHC-stained and ISH-stained slides were viewed and the images captured using an Olympus BX53 light microscope (Tokyo, Japan). All IF IHC-stained slides were viewed and the images captured using an Olympus FV1200 confocal laser scanning microscope and processed with cellSens Dimension 1.11 software using two-dimensional deconvolution algorithm (Olympus).
Discussion
The identification of a subpopulation of CSCs within MDOTSCC expressing p63, a marker of squamous epithelium, that also expresses the CSC marker CD44 and the ESC markers SOX2, OCT4, SALL4 and pSTAT3 in this study is novel. To the best of our knowledge, this is the first report demonstrating a separate subpopulation of CSCs that express OCT4 exclusively within MDOTSCC. Whether this represents a separate CSC or a ‘normal’ stem cell recruited into the tumour remains to be conclusively determined. The findings of at least two putative CSC subpopulations within MDOTSCC is novel and may potentially be explained by previous assumptions of these cells being at the invasive tumour front and thus in the transitory process of migrating from the TNs,7 suggesting that the former originate from the latter. Alternatively, there are two distinct stem cell subpopulations within OTSCC, each playing a critical role in carcinogenesis.
Our intriguing findings of the cytoplasmic localisation of OCT4, SALL4, SOX2 and NANOG are consistent with reports on gliomas which have been attributed to disease specificity,8 distinct from that of normal embryonic development. This observation merits further investigation.
Future work including characterisation of CSCs in well and poorly differentiated OTSCC using the same markers and the identification of their regulatory pathway(s) may uncover novel therapeutic targets.
Acknowledgments
The authors thank Ms Liz Jones and Dr Andrea Mikulasova of the Gillies McIndoe Research Institute for their assistance in immunohistochemical (IHC) and in situ hybridisation (ISH) staining and the processing of tissues for NanoString analysis, respectively.
Footnotes
TI and STT equal senior authors.
Aspects of this work were presented at the Plastic Surgery Congress, Brisbane, Australia, 6–10 May 2015 and the work was awarded the ANZHNCS Research Foundation Prize at the Tri-Society Head and Neck Oncology Meeting, Darwin, Australia, 14–16 August 2014.
Contributors RB, TI and STT formulated the study hypothesis. RB, TI, HDB, PFD and STT designed the study. RB, HDB, HHY and TI interpreted the IHC data. HHY and TI interpreted the ISH data. RB, TI and STT drafted the manuscript. All authors approved the manuscript.
Funding RB was supported by the Kristen Deane Scholarship, a Faculty of Medicine Scholarship from the University of Otago, and a grant from Pub Charity. TI was supported, in part, by the Foundation for Surgery ANZ Journal of Surgery Scholarship, Royal Australasian College of Surgeons. This work was funded, in part, by the Genesis Oncology Trust.
Competing interests None declared.
Ethics approval Central Regional Health and Disability Ethics Committee (reference no: 12/CEN/74).
Provenance and peer review Not commissioned; externally peer reviewed.