Abstract
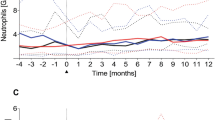
Little information is available on long-term immune reconstitution after therapy with alemtuzumab in B-CLL patients. We present long-term follow-up data for blood lymphocyte subsets analysed by flow cytometry in previously untreated B-CLL patients who received alemtuzumab subcutaneously as first-line therapy. All lymphoid subsets were significantly (P<0.001) and profoundly reduced; the median end-of-treatment counts for CD4+, CD8+, CD3−56+ (natural killer (NK)), CD3+56+ (NK–T) and CD19+5− (normal B) cells were 43, 20, 4, 1 and 8 cells/μl, respectively. The median cell count of all subsets remained at <25% of the baseline values for >9 months post-treatment. CD4+ and CD8+ levels in blood had reached >100 cells/μl in >50% of the patients at 4 months after the end of treatment. One patient had a cytomegalovirus reactivation and one patient developed Pneumocystis carinii pneumonia during therapy. No opportunistic or other major infections were recorded during unmaintained, long-term follow-up. There was no correlation between the cumulative dose of alemtuzumab and the severity or length of immunosuppression. CD52− T-cell subsets occurred during the treatment and comprised >80% of all CD4+ and CD8+ cells in the blood at the end of therapy. These subpopulations declined gradually during unmaintained follow-up. The relationship between these observations and the safety/antitumour effects of alemtuzumab is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Johnson S, Smith AG, Loffler H, Osby E, Juliusson G, Emmerich B et al. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet 1996; 347: 1432–1438.
Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukaemia. N Engl J Med 2000; 343: 1750–1757.
Montserrat E . Current and developing chemotherapy for CLL. Med Oncol 2002; 19 (Suppl): S11–S19.
Rai KR, Freter CE, Mercier RJ, Cooper MR, Mitchell BS, Stadtmauer EA et al. Alemtuzumab in previously treated chronic lymphocytic leukaemia patients who also had received fludarabine. J Clin Oncol 2002; 20: 3891–3897.
Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J et al. Therapeutic role of alemtuzumab (CAMPATH-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002; 99: 3554–3561.
Osterborg A, Dyer MJ, Bunjes D, Pangalis GA, Bastion Y, Catovsky D et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukaemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukaemia. J Clin Oncol 1997; 15: 1567–1574.
Osterborg A, Fassas AS, Anagnostopoulos A, Dyer MJ, Catovsky D, Mellstedt H . Humanized CD52 monoclonal antibody CAMPATH-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol 1996; 93: 151–153.
Gilleece MH, Dexter TM . Effect of CAMPATH-1H antibody on human haematopoietic progenitors in vitro. Blood 1993; 82: 807–812.
Lundin J, Kimby E, Bjorkholm M, Broliden PA, Celsing F, Hjalmar V et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (CAMPATH-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukaemia (B-CLL). Blood 2002; 100: 768–773.
Hertenstein B, Wagner B, Bunjes D, Duncker C, Raghavachar A, Arnold R et al. Emergence of CD52-, phosphatidylinositolglycan-anchor-deficient T lymphocytes after in vivo application of CAMPATH-1H for refractory B-cell non-Hodgkin lymphoma. Blood 1995; 86: 1487–1492.
Rawstron AC, Rollinson SJ, Richards S, Short MA, English A, Morgan GJ et al. The PNH phenotype cells that emerge in most patients after CAMPATH-1H therapy are present prior to treatment. Br J Haematol 1999; 107: 148–153.
Osterborg A, Werner A, Halapi E, Lundin J, Harmenberg U, Wigzell H et al. Clonal CD8+ and CD52− T cells are induced in responding B cell lymphoma patients treated with CAMPATH-1H (anti-CD52). Eur J Haematol 1997; 58: 5–13.
Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S et al. National Cancer Institute-Sponsored Working Group guidelines for chronic lymphocytic leukaemia: revised guidelines for diagnosis and treatment. Blood 1996; 87: 4990–4997.
Rai KR, Sawitsky A, Cronkite EP, Chanana AD, Levy RN, Pasternack BS . Clinical staging of chronic lymphocytic leukaemia. Blood 1975; 46: 219–234.
Lundin J, Osterborg A, Brittinger G, Crowther D, Dombret H, Engert A et al. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin's lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin's Lymphoma. J Clin Oncol 1998; 16: 3257–3263.
Perkins JG, Flynn JM, Howard RS, Byrd JC . Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukaemia and small lymphocytic lymphoma: implications for clinical trials in this patient population. Cancer 2002; 94: 2033–2039.
Vuillier F, Tortevoye P, Binet JL, Dighiero G . CD4, CD8 and NK subsets in B-CLL. Nouv Rev Fr Hematol 1988; 30: 331–334.
Reyes E, Prieto A, Carrion F, Garcia-Suarez J, Esquivel F, Alvarez-Mon M . Morphological variants of leukemic cells in B chronic lymphocytic leukaemia are associated with different T cell and NK cell abnormalities. Am J Hematol 1997; 55: 175–182.
Brett S, Baxter G, Cooper H, Johnston JM, Tite J, Rapson N . Repopulation of blood lymphocyte sub-populations in rheumatoid arthritis patients treated with the depleting humanized monoclonal antibody, CAMPATH-1H. Immunology 1996; 88: 13–19.
Davison GM, Novitzky N, Kline A, Thomas V, Abrahams L, Hale G et al. Immune reconstitution after allogeneic bone marrow transplantation depleted of T cells. Transplantation 2000; 69: 1341–1347.
Parreira A, Smith J, Hows JM, Smithers SA, Apperley J, Rombos Y et al. Immunological reconstitution after bone marrow transplant with CAMPATH-1 treated bone marrow. Clin Exp Immunol 1987; 67: 142–150.
Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of CAMPATH-1H in delaying immune reconstitution. Blood 2002; 99: 4357–4363.
Isaacs JD, Watts RA, Hazleman BL, Hale G, Keogan MT, Cobbold SP et al. Humanised monoclonal antibody therapy for rheumatoid arthritis. Lancet 1992; 340: 748–752.
Coles AJ, Wing M, Smith S, Coraddu F, Greer S, Taylor C et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 1999; 354: 1691–1695.
Acknowledgements
The secretarial help of Ms Gerd Ståhlberg is highly appreciated. This study was approved by the Ethics Committee of Karolinska Institutet, and conducted in accordance with the declaration of Helsinki. The study was supported by the Swedish Cancer Society, the Cancer Society in Stockholm, the Torsten and Ragnar Söderberg Foundation, Schering AG, Berlin, Germany and Ilex Oncology Inc., San Antonio, TX, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundin, J., Porwit-MacDonald, A., Rossmann, E. et al. Cellular immune reconstitution after subcutaneous alemtuzumab (anti-CD52 monoclonal antibody, CAMPATH-1H) treatment as first-line therapy for B-cell chronic lymphocytic leukaemia. Leukemia 18, 484–490 (2004). https://doi.org/10.1038/sj.leu.2403258
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403258
Keywords
This article is cited by
-
Review of the Clinical Pharmacokinetics and Pharmacodynamics of Alemtuzumab and Its Use in Kidney Transplantation
Clinical Pharmacokinetics (2018)
-
Phase I–II study of lenalidomide and alemtuzumab in refractory chronic lymphocytic leukemia (CLL): effects on T cells and immune checkpoints
Cancer Immunology, Immunotherapy (2017)
-
Dose modification of alemtuzumab in combination with dexamethasone, cytarabine, and cisplatin in patients with relapsed or refractory peripheral T-cell lymphoma: analysis of efficacy and toxicity
Investigational New Drugs (2012)
-
Vaccination with dendritic cells loaded with tumor apoptotic bodies (Apo-DC) in patients with chronic lymphocytic leukemia: effects of various adjuvants and definition of immune response criteria
Cancer Immunology, Immunotherapy (2012)
-
Virus reactivations and serology patterns following first-line therapy with alemtuzumab or fludarabine-based combination therapy in patients with chronic lymphocytic leukemia
Blood Cancer Journal (2011)