Abstract
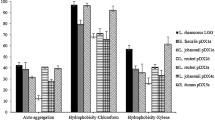
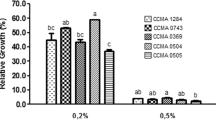
Strains of Lactobacillus spp., isolated from sourdough and olive brines (seven strains), and three human isolates were screened for their antagonistic activity in coculture against the ureolytic pathogen Yersinia enterocolitica. A general reduction in the pathogen population was observed after 6 h when each Lactobacillus strain was cocultured with the pathogen at a ratio of 100:1 cfu ml−1, causing an almost complete inhibition of urease activity. Strains were also screened for their performances in in vitro tests such as adhesion ability to Caco-2 cells, tolerance to low pH, bile salts, and simulated digestion, which enabled the differences between strains to be highlighted. Three strains, L. paracasei IMPC2.1 and L. plantarum ITM21B and ITM5BG, met the main criteria for selecting effective probiotics: the ability to inhibit the pathogen Y. enterocolitica and, consequently, its urease activity (ITM21B); survival of simulated digestion (ITM21B and IMPC2.1); strong adhesion ability to enterocytes and good survival at low pH and in the presence of bile salts (ITM5BG and IMPC2.1).


Similar content being viewed by others
References
Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y (1998) Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol 93:2097–2101
Bengmark S (1996) Econutrition and health maintenance–a new concept to prevent GI inflammation, ulceration and sepsis. Clin Nutr 15:1–10
Bhaduri S, Wesley IV, Bush EJ (2005) Prevalence of pathogenic Yersinia enterocolitica strains in pigs in the United States. Appl Environ Microbiol 71:7117–7121
Brassart D, Shiffrin EJ (1997) The use of probiotics to reinforce mucosal defence mechanism. Trends Food Sci Tech 8:321–326
Corsetti A, Lavermicocca P, Morea M, Baruzzi F, Tosti N, Gobbetti M (2001) Phenotypic and molecular identification and clustering of lactic acid bacteria and yeasts from wheat (species Triticum durum and Triticum aestivum) sourdoughs of southern Italy. Int J Food Microbiol 64:95–104
Cross ML (2002) Microbes versus microbes: immune signals generated by probiotic lactobacilli and their role in protection against microbial pathogens. FEMS Immunol Med Microbiol 34:245–253
De Koning-Ward TF, Robins-Browne RM (1995) Contribution of urease to acid tolerance in Yersinia enterocolitica. Infect Immun 63:3790–3795
Eaton KA, Brooks CL, Morgan CL, Kracowka S (1991) Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun 59:2470–2475
Elo S, Saxelin M, Salminen S (1991) Attachment of Lactobacillus casei K strain GG to human colon carcinoma cell line Caco-2: comparison with other dairy strains. Lett Appl Microbiol 13:154–156
Fernández MF, Boris S, Barbès C (2003) Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J Appl Microbiol 94:449–455
Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S (2001) Probiotics: effects on immunity. Am J Clin Nutr 73:444s–450s
Janssen M, Geeraerd AH, Logist F, De Visscher Y, Vereecken KM, Debevere J, Devlieghere F, Van Impe JF (2006) Modelling Yersinia enterocolitica inactivation in coculture experiments with Lactobacillus sakei as based on pH and lactic acid profiles. Int J Food Microbiol 111:59–72
Lavermicocca P (2006) Highlights on new food research. Dig Liver Dis 38(Suppl 2):S295–S299
Lavermicocca P, Valerio F, Lonigro SL, De Angelis M, Morelli L, Callegari ML, Rizzello CG, Visconti A (2005) Study of adhesion and survival of lactobacilli and bifidobacteria on table olives with the aim of formulating a new probiotic food. Appl Environ Microbiol 71:4233–4240
Lavermicocca P, Gobbetti M, Corsetti A, Caputo L (1998) Characterization of lactic acid bacteria isolated from olive phylloplane and table olive brines. Ital J Food Sci 10:27–39
Mobley HL, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases. Microbiol Rev 59:451–480
Raibaud P, Galpin JV, Ducluzeau R, Mocquot F, Oliver G. (1973) Le genre Lactobacillus dans le tube digestif du rat. II. Caractères de souches hétérofermentaires isolés de rats “holo” et “gnotoxéniques. Ann Microbiol 124:223–235
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130:396S–402S
Saarela M, Lahteenmaki L, Crittenden R, Salminen S, Mattila-Sandholm T (2002) Gut bacteria and health foods—the European perspective. Int J Food Microbiol 78:99–117
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17:741–754
Sgouras D, Maragkoudakis P, Petraki K, Martinez-Gonzalez B, Eriotou E, Michopoulos S, Kalantzopoulos G, Tsakalidou E, Mentis A (2004) In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol 70:518–526
Valerio F, De Bellis P, Lonigro SL, Morelli L, Visconti A, Lavermicocca P (2006) In vitro and in vivo survival and transit tolerance of potentially probiotic strains carried by artichokes in the gastrointestinal tract. Appl Environ Microbiol 72:3042–3045
Vereecken KM, Devlieghere F, Bockstaele A., Debevere J, Van Impe JF (2003) A model for lactic acid-induced inhibition of Yersinia enterocolitica in mono- and coculture with Lactobacillus sakei. Food Microbiol 20:701–713
Acknowledgments
This work was supported by Fondazione Cassa di Risparmio di Puglia (Bari, Italy) and by the Italian Ministry for University and Research (art. 12 D.M. 593/2000, D.D. 3300, 22 December 2005, tema 2) Project “Ortobiotici pugliesi” (D.M. 28830). The authors would like to thank Anthony Green for revising the English in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lavermicocca, P., Valerio, F., Lonigro, S.L. et al. Antagonistic Activity of Potential Probiotic Lactobacilli Against the Ureolytic Pathogen Yersinia enterocolitica . Curr Microbiol 56, 175–181 (2008). https://doi.org/10.1007/s00284-007-9069-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-007-9069-5