Abstract
Background
In sudden, unexpected, non-traumatic death in young individuals, structural abnormalities of the heart are frequently identified at autopsy. However, the findings may be unspecific and cause of death may remain unclear. A significant proportion of these cases are most likely caused by inherited cardiac diseases, and the cases are categorized as sudden cardiac death (SCD). The purpose of this study was to explore the added diagnostic value of genetic testing by next-generation sequencing (NGS) of a broad gene panel, as a supplement to the traditional forensic investigation in cases with non-diagnostic structural abnormalities of the heart.
Methods and results
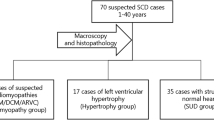
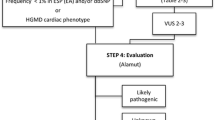
We screened 72 suspected SCD cases (<50 years) using the HaloPlex Target Enrichment System (Agilent) and NGS (Illumina MiSeq) for 100 genes previously associated with inherited cardiomyopathies and channelopathies. Fifty-two cases had non-diagnostic structural cardiac abnormalities and 20 cases, diagnosed with a cardiomyopathy post-mortem (ARVC = 14, HCM = 6), served as comparators. Fifteen (29 %) of the deceased individuals with non-diagnostic findings had variants with likely functional effects based on conservation, computational prediction, allele-frequency and supportive literature. The corresponding frequency in deceased individuals with cardiomyopathies was 35 % (p = 0.8).
Conclusion
The broad genetic screening revealed variants with likely functional effects at similar high rates, i.e. in 29 and 35 % of the suspected SCD cases with non-diagnostic and diagnostic cardiac abnormalities, respectively. Although the interpretation of broad NGS screening is challenging, it can support the forensic investigation and help the cardiologist’s decision to offer counselling and clinical evaluation to relatives of young SCD victims.

Similar content being viewed by others
References
Risgaard B, Winkel BG, Jabbari R, Behr ER, Ingemann-Hansen O, Thomsen JL, Ottesen GL, Gislason GH, Bundgaard H, Haunso S, Holst AG, Tfelt-Hansen J (2014) Burden of sudden cardiac death in persons aged 1 to 49 years: nationwide study in denmark. Circ Arrhythm Electrophysiol 7(2):205–211. doi:10.1161/CIRCEP.113.001421
www.who.int World Health Organization. International Classification of Disease 10 (ICD-10). http://www.who.int/classifications/icd/en/. Accessed 01 Jul 2015
Puranik R, Chow CK, Duflou JA, Kilborn MJ, McGuire MA (2005) Sudden death in the young. Heart Rhythm 2(12):1277–1282. doi:10.1016/j.hrthm.2005.09.008
Ferrero‐Miliani L, Holst AG, Pehrson S, Morling N, Bundgaard H (2010) Strategy for clinical evaluation and screening of sudden cardiac death relatives. Fundamental Clin Pharmacol 24(5):619–635. doi:10.1111/j.1472-8206.2010.00864.x
Behr ER, Dalageorgou C, Christiansen M, Syrris P, Hughes S, Esteban MTT, Rowland E, Jeffery S, McKenna WJ (2008) Sudden arrhythmic death syndrome: familial evaluation identifies inheritable heart disease in the majority of families. Eur Heart J 29(13):1670–1680. doi:10.1093/eurheartj/ehn219
Papadakis M, Raju H, Behr ER, Noronha SVD, Spath N, Kouloubinis A, Sheppard MN, Sharma S (2013) Sudden cardiac death with autopsy findings of uncertain significance potential for erroneous interpretation. Circ Arrhythm Electrophysiol 6(3):588–596. doi:10.1161/CIRCEP.113.000111
Skinner JR, Crawford J, Smith W, Aitken A, Heaven D, Evans C-A, Hayes I, Neas KR, Stables S, Koelmeyer T, Denmark L, Vuletic J, Maxwell F, White K, Yang T, Roden DM, Leren TP, Shelling A, Love DR (2011) Prospective, population-based long QT molecular autopsy study of postmortem negative sudden death in 1 to 40 year olds. Heart Rhythm 8(3):412–419. doi:10.1016/j.hrthm.2010.11.016
Winkel BG, Larsen MK, Berge KE, Leren TP, Nissen PH, Olesen MS, Hollegaard MV, Jespersen T, Yuan L, Nielsen N, Haunsø S, Svendsen JH, Wang Y, Kristensen IB, Jensen HK, Tfelt-Hansen J, Banner J (2012) The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol 23(10):1092–1098. doi:10.1111/j.1540-8167.2012.02371.x
Tester DJ, Medeiros-Domingo A, Will ML, Haglund CM, Ackerman MJ (2012) Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc 87(6):524–539. doi:10.1016/j.mayocp.2012.02.017
Hertz CL, Christiansen SL, Ferrero-Miliani L, Fordyce SL, Dahl M, Holst AG, Ottesen GL, Frank-Hansen R, Bundgaard H, Morling N (2014) Next-generation sequencing of 34 genes in sudden unexplained death victims in forensics and in patients with channelopathic cardiac diseases. Int J Legal Med. doi:10.1007/s00414-014-1105-y
Allegue C, Gil R, Blanco-Verea A, Santori M, Rodríguez-Calvo M, Concheiro L, Carracedo Á, Brion M (2011) Prevalence of HCM and long QT syndrome mutations in young sudden cardiac death-related cases. Int J Legal Med 125(4):565–572. doi:10.1007/s00414-011-0572-7
Zhang M, Tavora F, Oliveira JB, Li L, Franco M, Fowler D, Zhao Z, Burke A (2012) PKP2 mutations in sudden death from arrhythmogenic right ventricular cardiomyopathy (ARVC) and sudden unexpected death with negative autopsy (SUDNA). Circ J 76(1):189–194. doi:10.1253/circj.CJ-11-0747
Larsen MK, Nissen PH, Berge KE, Leren TP, Kristensen IB, Jensen HK, Banner J (2012) Molecular autopsy in young sudden cardiac death victims with suspected cardiomyopathy. Forensic Sci Int 219(1–3):33–38. doi:10.1016/j.forsciint.2011.11.020
Hertz CL, Ferrero-Miliani L, Frank-Hansen R, Morling N, Bundgaard H (2014) A comparison of genetic findings in sudden cardiac death victims and cardiac patients: the importance of phenotypic classification. Europace. doi:10.1093/europace/euu210
Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DMY, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W (2010) Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia proposed modification of the task force criteria. Eur Heart J 31(7):806–814. doi:10.1093/eurheartj/ehq025
Maron BJ, Carney KP, Lever HM, Lewis JF, Barac I, Casey SA, Sherrid MV (2003) Relationship of race to sudden cardiac death in competitive athletes with hypertrophic cardiomyopathy. J Am Coll Cardiol 41(6):974–980. doi:10.1016/S0735-1097(02)02976-5
www.omim.org Online Mendelian Inheritance in Man. http://omim.org/. Accessed 01 Mar 2014
Kent W, Sugnet C, Furey T, Roskin K, Pringle T, Zahler A, Haussler D (2002) The human genome browser at USCS. Genome Res 12(6):996–1006. doi:10.1101/gr.229102
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler Transform. Bioinformatics 25:1754–1760. doi:10.1093/bioinformatics/btp324
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, Genome Project Data Processing S (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25(16):2078–2079. doi:10.1093/bioinformatics/btp352
www.evs.gs.washington.edu/EVS Exome Variant Server. http://evs.gs.washington.edu/EVS/. Accessed 02 Jan 2014
www.ncbi.nlm.nih.gov National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov/. Accessed 10 Feb 2014
Stenson P, Ball E, Mort M, Phillips A, Shiel J, Thomas N, Abeysinghe S, Krawczak M, Copper D (2003) The Human Gene Mutation Database (HGMD). Hum Mutat 21:577–581. doi:10.1002/humu.10212
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med : Off J Am Coll Med Genet 17(5):405–423. doi:10.1038/gim.2015.30
Ng D, Johnston JJ, Teer JK, Singh LN, Peller LC, Wynter JS, Lewis KL, Cooper DN, Stenson PD, Mullikin JC, Biesecker LG, Program NIHISCCS (2013) Interpreting secondary cardiac disease variants in an exome cohort. Circ Cardiovasc Genet 6(4):337–346. doi:10.1161/CIRCGENETICS.113.000039
Dorschner MO, Amendola LM, Turner EH, Robertson PD, Shirts BH, Gallego CJ, Bennett RL, Jones KL, Tokita MJ, Bennett JT, Kim JH, Rosenthal EA, Kim DS, National Heart L, Blood Institute Grand Opportunity Exome Sequencing P, Tabor HK, Bamshad MJ, Motulsky AG, Scott CR, Pritchard CC, Walsh T, Burke W, Raskind WH, Byers P, Hisama FM, Nickerson DA, Jarvik GP (2013) Actionable, pathogenic incidental findings in 1,000 participants' exomes. Am J Hum Genet 93 (4):631–640. doi:10.1016/j.ajhg.2013.08.006
Mathieson I, McVean G (2012) Differential confounding of rare and common variants in spatially structured populations. Nat Genet 44(3):243–246. doi:10.1038/ng.1074
Lohmueller KE, Sparso T, Li Q, Andersson E, Korneliussen T, Albrechtsen A, Banasik K, Grarup N, Hallgrimsdottir I, Kiil K, Kilpelainen TO, Krarup NT, Pers TH, Sanchez G, Hu Y, Degiorgio M, Jorgensen T, Sandbaek A, Lauritzen T, Brunak S, Kristiansen K, Li Y, Hansen T, Wang J, Nielsen R, Pedersen O (2013) Whole-exome sequencing of 2,000 Danish individuals and the role of rare coding variants in type 2 diabetes. Am J Hum Genet 93(6):1072–1086. doi:10.1016/j.ajhg.2013.11.005
Team RC (2014) R: a language and environment for statistical computing. Available via R Foundation for Statistical Computing. http://www.R-project.org
Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, Gottardo R, Hahne F, Hansen KD, Irizarry RA, Lawrence M, Love MI, MacDonald J, Obenchain V, Oles AK, Pages H, Reyes A, Shannon P, Smyth GK, Tenenbaum D, Waldron L, Morgan M (2015) Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods 12(2):115–121. doi:10.1038/nmeth.3252
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5(10):R80. doi:10.1186/gb-2004-5-10-r80
Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M (1996) Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 94(5):983–991
Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ (2008) Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol 52(25):2175–2187. doi:10.1016/j.jacc.2008.09.019
Bagnall RD, Das KJ, Duflou J, Semsarian C (2014) Exome analysis-based molecular autopsy in cases of sudden unexplained death in the young. Heart Rhythm 11(4):655–662. doi:10.1016/j.hrthm.2014.01.017
Narula N, Tester DJ, Paulmichl A, Maleszewski JJ, Ackerman MJ (2014) Post-mortem whole exome sequencing with gene-specific analysis for autopsy-negative sudden unexplained death in the young: a case series. Pediatr Cardiol. doi:10.1007/s00246-014-1082-4
Millat G, Chanavat V, Rousson R (2014) Evaluation of a new NGS method based on a custom AmpliSeq library and Ion Torrent PGM sequencing for the fast detection of genetic variations in cardiomyopathies. Clin Chim Acta; Int J Clin Chem 433:266–271. doi:10.1016/j.cca.2014.03.032
Sikkema-Raddatz B, Johansson LF, de Boer EN, Almomani R, Boven LG, van den Berg MP, van Spaendonck-Zwarts KY, van Tintelen JP, Sijmons RH, Jongbloed JD, Sinke RJ (2013) Targeted next-generation sequencing can replace Sanger sequencing in clinical diagnostics. Hum Mutat 34(7):1035–1042. doi:10.1002/humu.22332
Mook ORF, Haagmans MA, Soucy J-F, van de Meerakker JBA, Baas F, Jakobs ME, Hofman N, Christiaans I, Lekanne Deprez RH, Mannens MMAM (2013) Targeted sequence capture and GS-FLX Titanium sequencing of 23 hypertrophic and dilated cardiomyopathy genes: implementation into diagnostics. J Med Genet 50(9):614–626. doi:10.1136/jmedgenet-2012-101231
Zimmerman RS, Cox S, Lakdawala NK, Cirino A, Mancini-DiNardo D, Clark E, Leon A, Duffy E, White E, Baxter S, Alaamery M, Farwell L, Weiss S, Seidman CE, Seidman JG, Ho CY, Rehm HL, Funke BH (2010) A novel custom resequencing array for dilated cardiomyopathy. Genet Med : Off J Am Coll Med Genet 12(5):268–278. doi:10.1097/GIM.0b013e3181d6f7c0
Roux-Buisson N, Gandjbakhch E, Donal E, Probst V, Deharo J, Chevalier P, Klug D, Masencal N, Delacretaz E, Cosnay P, Scanu P, Extramiana F, Keller D, Hidden-Lucet F, Trapani J, Fouret P, Frank R, Fressart V, Fauré J (2014) Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: results of a systematic screening. Heart Rhythm 11(11):1999–2009. doi:10.1016/j.hrthm.2014.07.020
Abraham RL, Yang T, Blair M, Roden DM, Darbar D (2010) Augmented potassium current is a shared phenotype for two genetic defects associated with familial atrial fibrillation. J Mol Cell Cardiol 48(1):181–190. doi:10.1016/j.yjmcc.2009.07.020
Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P (2010) Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3(4):374–385. doi:10.1161/CIRCGENETICS.109.930867
Stallmeyer B, Zumhagen S, Denjoy I, Duthoit G, Hebert JL, Ferrer X, Maugenre S, Schmitz W, Kirchhefer U, Schulze-Bahr E, Guicheney P, Schulze-Bahr E (2012) Mutational spectrum in the Ca(2+)—activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat 33(1):109–117. doi:10.1002/humu.21599
Nielsen NH, Winkel BG, Kanters JK, Schmitt N, Hofman-Bang J, Jensen HS, Bentzen BH, Sigurd B, Larsen LA, Andersen PS, Haunso S, Kjeldsen K, Grunnet M, Christiansen M, Olesen SP (2007) Mutations in the Kv1.5 channel gene KCNA5 in cardiac arrest patients. Biochem Biophys Res Commun 354(3):776–782. doi:10.1016/j.bbrc.2007.01.048
Gonzalez-Garay ML, McGuire AL, Pereira S, Caskey CT (2013) Personalized genomic disease risk of volunteers. Proc Natl Acad Sci U S A 110(42):16957–16962. doi:10.1073/pnas.1315934110
Hassel D, Dahme T, Erdmann J, Meder B, Huge A, Stoll M, Just S, Hess A, Ehlermann P, Weichenhan D, Grimmler M, Liptau H, Hetzer R, Regitz-Zagrosek V, Fischer C, Nurnberg P, Schunkert H, Katus HA, Rottbauer W (2009) Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat Med 15(11):1281–1288. doi:10.1038/nm.2037
Hertz CL, Christiansen SL, Larsen MK, Dahl M, Ferrero-Miliani L, Weeke PE, Pedersen O, Hansen T, Grarup N, Ottesen GL, Frank-Hansen R, Banner J, Morling N (2015) Genetic investigations of sudden unexpected deaths in infancy using next-generation sequencing of 100 genes associated with cardiac diseases. Eur J Human Genet Accept. In press
Acknowledgments
The authors thank Francisc-Raul Kantor for bioinformatics support. For the screening of variants among Danish controls, we thank LuCamp, The Lundbeck Foundation Centre for Applied Medical Genomics in Personalized Disease Prediction, Prevention, and Care (www.lucamp.org), and the Novo Nordisk Foundation Center for Basic Metabolic Research, which is an independent Research Center at the University of Copenhagen partially supported by an unrestricted donation from the Novo Nordisk Foundation (www.metabol.ku.dk).
Funding sources
This work was supported by Ellen and Aage Andersen’s foundation and The A.P. Møller Foundation.
Conflic of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
C. L. Hertz and S. L. Christiansen have shared first authorship and contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 479 kb)
Rights and permissions
About this article
Cite this article
Hertz, C.L., Christiansen, S.L., Ferrero-Miliani, L. et al. Next-generation sequencing of 100 candidate genes in young victims of suspected sudden cardiac death with structural abnormalities of the heart. Int J Legal Med 130, 91–102 (2016). https://doi.org/10.1007/s00414-015-1261-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-015-1261-8