Abstract
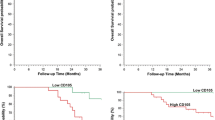
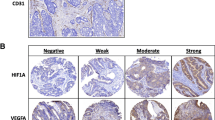
Tumor–node–metastasis (TNM) stage I colorectal cancer is commonly characterized by a good prognosis, with 5-year survival around 80–90%; nonetheless, it undergoes disease progression in a percentage of cases, although the causes of adverse clinical course still remain to be elucidated. In the present study, we analyzed and compared the immunohistochemical expression of the pro-angiogenic vascular endothelial growth factor (VEGF) as well as the microvessel density (MVD) in a series of 27 surgically resected colorectal carcinomas obtained from patients deceased because of disease progression and in a cohort of 25 colorectal cancers from patients still alive with no evidence of disease progression 5 years after the initial diagnosis. The prognostic value of VEGF expression and of MVD on the overall survival to colorectal cancer was investigated. A variable VEGF immunoexpression was demonstrated in all the analyzed cases. High VEGF expression was significantly more frequent among patients deceased of the disease. These patients also displayed significantly higher MVD counts in their cancer in comparison to the patients alive after 5 years from surgery. Moreover, both high VEGF expression and MVD appeared as significant negative prognostic markers related to a shorter overall survival to stage I colorectal carcinoma, with VEGF representing an independent variable at multivariate analysis. VEGF assessment might be used in order to select those patients with a higher progression risk and to submit them to adjuvant therapies useful to prevent adverse outcome.




Similar content being viewed by others
References
Landis SH, Murray T, Bolden S (1999) Cancer statistics. Cancer J Clin 49:8–31
Wiggers T, Arends JW, Schutter B et al (1988) A multivariate analysis of pathologic prognostic indicators in large bowel cancer. Cancer 61:386–389
Wu XC, Chen VW, Steele B et al (2001) Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group. Cancer 92:2547–2554
Di Gregorio C, Fante R, Roncucci L et al (1996) Clinical features, frequency and prognosis of Dukes’ A colorectal carcinoma: a population-based investigation. Eur J Cancer 32A:1957–1962
Di Gregorio C, Benfatti P, Losi L et al (2005) Incidence and survival of patients with Dukes’A (stages T1 and T2) colorectal carcinoma: a 15-year population-based study. Int J Colorectal Dis 20:147–154
Wichmann MW, Muller C, Hornung HM (2002) The Colorectal Cancer study Group. Results of long-term follow-up after curative resection of Dukes’ A colorectal cancer. World J Surg 26:732–736
Folkman J (1990) What is the evidence that tumour are angiogenesis dependent? J Natl Cancer Inst 82:4–6
Folkman J, Shing Y (1992) Angiogenesis. J Biol Chem 267:10931–10934
Folkman J (1995) Clinical applications of angiogenesis research. N Engl J Med 333:1757–1763
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumourigenesis. Cell 86:353–364
Ferrara N, Davis-Smith T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Fan F, Wey JS, McCarty MF et al (2005) Expression and function of vascular endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene 24:2647–2653
Cheifetz S, Bellon T, Cales C et al (1992) Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem 267:19027–19030
Burrows FJ, Derbyshire EJ, Tazzari PL et al (1995) Up-regulation of endoglin on vascular endothelial cells in human solid tumours: implications for diagnosis and therapy. Clin Cancer Res 1:1623–1634
Miller DW, Graulich W, Karges B (1999) Elevated expression of endoglin, a component of the TGF-β receptor complex, correlates with proliferation of tumour endothelial cells. Int J Cancer 81:568–572
Minhajat R, Mori D, Yamasaki F (2006) Endoglin (CD105) expression in angiogenesis of colon cancer: analysis using tissue microarrays and comparison with other endothelial markers. Virchows Arch 448:127–134
Saad RS, Liu YL, Nathan G et al (2004) Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol 17:197–203
Sobin LH, Wittekind C (eds) (2002) TNM classification of malignant tumors. International Union Against Cancer (UICC), 6th edn. Wiley-Liss, New York
Ponz de Leon M, Sassatelli R, Sacchetti C (1989) Familial aggregation of tumors in the three-year experience of a population-based colorectal cancer registry. Cancer Res 49:4344–4348
Ponz de Leon M, Benatti P, Percesepe A (1999) Epidemiology of cancer of the large bowel—the 12-year experience of a specialized registry in northern Italy. Ital J Gastroenterol Hepatol 31:10–18
Barresi V, Vitarelli E, Cerasoli S (2009) Semaphorin3A immunohistochemical expression in human meningiomas: correlation with the microvessel density. Virchows Arch 454:563–571
Barresi V, Tuccari G (2010) Increased ratio of vascular endothelial growth factor to semaphorin3A is a negative prognostic factor in human meningiomas. Neuropathology. doi:10.1111/j.1440-1789.2010.01105.x
Barresi V, Cerasoli S, Vitarelli E et al (2007) Density of microvessel positive for CD105 (endoglin) is related to prognosis in meningiomas. Acta Neuropathol 114:147–156
Barresi V, Cerasoli S, Tuccari G (2008) Correlative evidence that tumour cell-derived caveolin-1 mediates angiogenesis in meningiomas. Neuropathology 28:472–478
Torsney E, Charlton R, Parums D et al (2002) Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res 51:464–470
Fonsatti E, Del Vecchio L, Altomonte M (2001) Endoglin: an accessory component of the TGF-β-binding receptor complex with diagnostic, prognostic, and bio-immunotherapeutic potential in human malignancies. J Cell Physiol 188:1–7
Takahashi Y, Kitadai Y, Bucana CD et al (1995) Expression of vascular endothelial growth factor and its receptors, KDR, correlates with vascularity, metastasis and proliferation of human colon cancer. Cancer Res 55:3964–3968
Takahashi N, Haba A, Matsuno F et al (2001) Antiangiogenic therapy of established tumours in human skin/severe combined immunodeficiency mouse chimeras by anti-endoglin (CD105) monoclonal antibodies, and synergy between anti-endoglin antibody and cyclophosphamide. Cancer Res 61:7846–7854
Kang SM, Maeda K, Onoda N (1997) Combined analysis of p53 and vascular endothelial growth factor expression in colorectal carcinoma for determination of tumour vascularity and liver metastasis. Int J Cancer 74:502–507
Dassoulas K, Gazouli M, Theodoropoulos G (2010) Vascular endothelial growth factor and endoglin expression in colorectal cancer. J Cancer Res Clin Oncol 136:703–708
Ishigami SI, Arii S, Furutani M et al (1998) Predictive value of vascular endothelial growth factor (VEGF) for metastasis and prognosis of human colorectal cancer. Br J Cancer 78:1379–1384
Weidner N, Semple JP, Welch WR (1991) Tumour angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Shih T, Lindley C (2006) Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 28:1779–1802
Bose D, Meric-Bernstam F, Hofstetter W et al (2010) Vascular endothelial growth factor targeted therapy in the perioperative setting: implications for patient care. Lancet Oncol 11:373–382
WangY FD, Vanderlaan M et al (2004) Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 7:335–345
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685–693
Gerber HP, Ferrara N (2005) Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 65:671–680
Uneda S, Toi H, Tsujie T et al (2009) Anti-endoglin monoclonal antibodies are effective for suppressing metastasis and the primary tumours by targeting tumour vasculature. Int J Cancer 125:1446–1453
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barresi, V., Di Gregorio, C., Regiani-Bonetti, L. et al. Stage I colorectal carcinoma: VEGF immunohistochemical expression, microvessel density, and their correlation with clinical outcome. Virchows Arch 457, 11–19 (2010). https://doi.org/10.1007/s00428-010-0933-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-0933-5