Abstract
With the advent of new therapeutic modalities, the treatment options for oncologists can vary greatly depending upon the aggressiveness of the patient's cancer. Patients may receive no therapy, adjuvant therapy, aggressive adjuvant therapy (taxane based), monoclonal antibody therapy (e.g. Herceptin) or bone marrow transplantation. It is now mandatory to determine accurate prognostic patient profiles at diagnosis and during therapy to determine who would benefit most from a particular therapeutic regimen or to determine who should be shifted into more aggressive therapy. We now have ultra-sensitive methods of tumor cell detection that can determine the presence of minimal residual cancer (MRC) in marrow, stem cell product (SCP) and lymph node to help create these prognostic profiles. The author has conducted a critical review of the literature regarding the type of testing used to detect MRC, the incidence of MRC in marrow, SCP, and lymph node, and the clinical significance of MRC at diagnosis and during therapy.
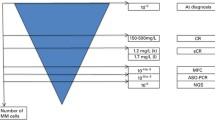
To date it is now clear that immunohistochemistry is a very useful diagnostic tool with adequate sensitivity to detect MRC. The presence of MRC at diagnosis in marrow and/or lymph node is associated with a poor prognosis for a number of disorders including breast cancer, neuroblastoma, gastrointestinal tumors, and lung cancer. In addition, the presence of MRC during therapy in marrow and/or SCP is associated with a very poor prognosis for patients with breast cancer. The use of testing for MRC in the patient provides prognostic information that may be of use to the oncologist.
Similar content being viewed by others
References
Shpall EJ, Jones RB, Bearman SI, et al.: Transplantation of enriched CD-34-positive autologous marrow into breast cancer patients following high-dose chemotherapy: Influence of CD34-positive peripheral-blood progenitors and growth factors on engraftment. J Clin Oncol 12: 28–36, 1994
Moss TJ, Cooper B, Kennedy MJ, Meagher R, Herzig R, Copelan E, Lazarus H: The prognostic value of immunocytologic (IHC) analysis on bone marrow (BM) taken from patients with stage IV breast cancer undergoing autologous transplant (ABMT) therapy. Blood 88:10 Suppl 1: 128, 1996
Ross AA, Cooper BW, Lazarus HM, Mackay W, Moss TJ, Ciobanu N, Tallman MS, Kennedy MJ, Davidson NE, Sweet D, Winter C, Akard L, Jansen J, Copelan E, Meagher RC, Herzig RH, Klumpp TR, Kahn DG, Warner NE: Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood 82: 2605–10, 1993
Pantel K, Moss TJ: Meeting report: First international ISHAGE symposium on minimal residual cancer. J Hematoth 5: 511–7, 1996
Noga SJ, Kennedy MJ, Valone F, Van Vlasselaer P, Moss TJ: Density adjusted cell sorting (DACS) combined with negative CD45 enrichment of hematopoietic stem cell (HSC) harvests increases the sensitivity of immuno-cytochemical (IHC) detection for breast cancer. Proc Amer Soc of Clin Oncol 16: 102a, 1995
Mattano LA, Jr, Moss TJ, Emerson SG: Sensitive detection of rare circulating neuroblastoma cells by the reverse transcriptase-polymerase chain reaction. Cancer Res 52: 4701–4705, 1992
Fields K, Elfenbein G, Trudeau WL, Perkins JB, Janssen WE, Moscinski LC: Clinical significance of bone marrow metastases as detected using polymerase chain reaction in patients with breast cancer undergoing high-dose chemotherapy and autologous bone marrow transplantation. J Clin Oncol 14: 1868–76, 1996
Boel P, Wildmann C, Sensi ML, Brasseur R, Renauld J, Coulie P, Boon T, van der Bruggen P: BAGE: A new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity 2: 167–75, 1995
Miyajima Y, Kato K, Numata S, Kudo K, Horibe K: Detection of neuroblastoma cells in bone marrow and peripheral blood at diagnosis by the reverse transcriptase-polymerase chain reaction for tyrosine hydroxylase mRNA. Cancer 75: 2757–61, 1995
Bostick PJ, Chatterjee S, Chi DD, Huynh KT, Giuliano AE, Cote R, Hoon DS: Limitations of specific reverse-transcriptase polymerase chain reaction markers in the detection of metastases in lymph nodes and blood of breast cancer patients. J Clin Oncol 16: 2632–40, 1998
Zippelius A, Kufer P, Honold G, Kollermann MW, Oberneder R, Schlimok G, Riethmuller G, Pantel K: Limitations of reverse-transcriptase polymerase chain reaction analyses for detection of micrometastatic epithelial cancer cells in bone marrow. J Clin Oncol 15: 2701–8, 1997
Salmon SE, Hamburger AW, Soehnlen B, Durie BGM, Alberts DS, Moon TE: Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. New Eng J Med 298: 1321, 1978
Sharp JG, Kessinger A, Mann S: Outcome of high-dose therapy and autologous transplantation in non-Hodgkin's lymphoma based on the presence of tumor in the marrow or infused hematopoietic harvest. J Clin Oncol 14: 214–9, 1996
Sharp JG, Kessinger A, Vaughan WP, Mann S: Detection and clinical significance of minimal tumor cell contamination of peripheral stem cell harvests. Int J Cell Cloning 10(Suppl 1): 92, 1992
Passos-Coehlo JL, Ross AA, Moss TJ, Davis JM, Huelskamp A, Noga S, Davidson NE, Kenndy MJ: Absence of breast cancer cells in a single day peripheral blood progenitor cell (PBPC) collection following priming with cyclophosphamide and granulocyte-macrophage colony-stimulating factor (GM-CSF). Blood 84: 1138–43, 1995
Maruyama R, Sugio K, Mitsudomi T, Saitoh G, Ishida T, Sugimachi K: Relatioship between early recurrence and micrometastases in the lymph nodes of patients with stage I non-small-cell lung cancer. J Thorac Cardiovas Surg 114: 535–43, 1997
Giuliano AE, Kelemen PR: Sophisticated techniques detect obscure lymph node metastases in carcinoma of the breast. Cancer 83: 391–93, 1998
Moss TJ, Pantel K: Meeting report: Second international symposium on minimal residual cancer. J Hematoth (In Press, 1999)
Heiss MM, Aligayer H, Gruetzner KU, Babie R, Jauch KW, Schildberg FW: Clinical value of extended biologic staging by bone marrow micrometastases and tumor-associated proteases in gastric cancer. Ann Surg 226: 736–44, 1997
Woodhouse EC, Chuaqui RF, Liotta LA: General mechanisms of metastasis. Cancer 80(Suppl): 1529–37, 1997
Fox SB, Leek RD, Bliss J, Mansi JL, Gusterson B, Gatter KC, Harris AL: Association of tumor angiogenesis with bone marrow micrometastases in breast cancer patients. J Natl Cancer Inst 89: 1044–49, 1997
Bretton PR, Melamed MR, Fair WR, Cote RJ: Detection of occult micrometastases in the bone marrow of patients with prostate carcinoma. Prostate 25: 108–14, 1994
Mansi JL, Berger U, Easton D, McDonnell T, Redding WH, Gazet J-C, McKinna A, Powles TJ, Coombes RC: Micrometastases in bone marrow in patients with primary breast cancer: evaluation as an early predictor of bone metastases. Br Med J 295: 1093–96, 1987
Cote RJ, Beattie EJ, Chaiwan B, Shi SR, Harvey J, Chen SC, Sherrod AE, Groshen S, Taylor CR: Detection of occult bone marrow micrometastases in patients with operable lung carcinoma. Ann Surg 222: 415–23, 1995
Jauch KW, Heiss MM, Gruetzner U, Funke I, Pantel K, Babic R, Eissner HJ, Riethmuller G, Schildberg FW: Prognostic significance of bone marrow micrometastases in patients with gastric cancer. J Clin Oncol 14: 1810–17, 1996
Pantel K, Izbicki J, Passlick B, Angstwurm M, Haussinger K, Thetter O, Riethmuller G: Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet 347: 649–53, 1996
O'Sullivan GC, Collins JK, O'Brien F, Crowley B, Murphy K, Lee G, Shanahan F: Micrometastases in bone marrow of patients undergoing ‘curative’ surgery for gastrointestinal cancer. Gastroenterology 109: 1535–40, 1995
Moss TJ, Reynolds CP, Sather HN, Romansky SG, Hammond GD, Seeger RC: Prognostic value of immunocytologic detection of bone marrow metastases in neuroblastoma. New Engl J Med 324: 219–26, 1991
Cote RJ, Rosen PP, Lesser ML, Old LJ, Osborne MP: Prediction of early relapse in patients with operable breast cancer by detection of occult bone marrow micrometastases. J Clin Oncol 10: 1749–56
Pantel K, Schlimok G, Angstwurm M, Weckermann D, Werner S, Gath H, Passlick B, Izbicki JR, Riethmuller G: Methodological analysis of immunocytochemical screening for disseminated epithelail tumor cells in bone marrow. J Hematoth 3: 165–73, 1994
Pantel K: Detection of minimal disease in patients with solid tumors. J Hematoth 5: 359–67, 1996
Wood DP Jr, Banerjee M: Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J Clin Oncol 15: 3451–57, 1997
Diel IJ, Kaufmann M, Costa SD, Holle R, van Minckwitz, Solomayer EF, Kaul S, Bastert G: Micrometastatic breast cancer cells in bone marrow at primary surgery: Prognostic value in comparison with nodal status. J Natl Cancer Ins 88: 1652–58, 1996
Harbeck N, Untch M, Pache L, Eiermann W: Tumour cell detection in the bone marrow of breast cancer patients at primary therapy: Results of a 3-year median follow-up. Br J Cancer 69: 566–71, 1994
Redding WH, Coombes RC, Monaghan P, Clink HM, Imrie SF, Dearnaley DP: Detection of micrometastases in patients with primary breast cancer. Lancet ii: 1271–74, 1983
Ross AA, Muller G, Moss TJ, Kahn DF, Warner NE, Sweet DE, Louie, Sofdar S, Collins R, Fay J: Immunocytochemical detection of tumor cells in bone marrow specimens of ovarian carcinoma patients designated for high-dose chemotherapy and autologous BMT. Bone Marrow Transplant 15: 929–933, 1995
Schlimok G, Funke I, Bock B, Schweiberer B, Witte J, Riethmuller G: Epithelial tumor cells in bone marrow of patients with colorectal cancer: Immunocytochemical detection, phenotypic characterization, and prognostic significance. J Clin Oncol 8: 831, 1990
Fisher B, Redmond C, Fisher ER, et al.: Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. New Engl J Med 312: 674–81, 1985
Schoenfeld A, Luqmani Y, Sinnett HD, Shousha S, Coombes RC: Keratin 19 mRNA measurement to detect micrometastases in lymph nodes in breast cancer patients. Br J Cancer 74: 1639–42, 1996
Ishida K, Katsuyama T, Sugiyama A, Kawasaki S: Immunohistochemical evaluation of lymph node micrometastases from gastric carcinomas. Cancer 79: 1069–76, 1997
Liefers GJ, Cleton-Jansen AM, van de Velde CJ, Hermans J, van Krieken JH, Cornelisse CJ, Tollenaar RA: Micrometastases and survival in stage II colorectal cancer. N Engl J Med 339: 223–28, 1998
Salerno CT, Frizelle S, Niehans GA, Ho SB, Jakkula M, Kratzke RA, Maddaus MA: Detection of occult micrometastases in non-small cell lung carcinoma by reverse transcriptase-polymerase chain reaction. Chest 113: 1526–32, 1998
Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A, Passlick B, Broelsch CE, Pantel K: Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med 337: 1188–94, 1997
Giuliano AE, Jones RC, Brennan M, et al.: Sentinel lymphadenectomy in breast cancer. J Clin Oncol 15: 2345, 1997
Guenther JM, Krishnamoorthy M, Tan LR: Sentinel lymphadenectomy for breast cancer in a community managed care setting. Cancer J Sci Am 3: 336–40, 1997
Glass FL, Cottam JA, Reintgen DS, Fenske NA: Lymphatic mapping and sentinel node biopsy in the management of high-risk melanoma. J Am Acad Dermatol 39: 603–10, 1998
Pecora AL, Lazarus HM, Cooper B, Kennedy MJ, Umiel T, Meagher R, Herzig R, Copelan E, Prilutskaya M, Glassco J, Kahn DG, Moss TJ: Breast cancer contamination in peripheral blood stem cell (PBSC) collections association with bone marrow disease and type of mobilization. Blood 90:Suppl 1, 99a, 1997
Weaver CH, Moss TJ, Schwartzberg LS, Zhen B, et al.: High-dose chemotherapy in patients with breast cancer: Impact of infusing peripheral blood stem cells containing occult tumor cells. Bone Marrow Transplant 21: 1117–24, 1998
Gluck S, Ross AA, Layton TJ, Ostrander AB: Detection of breast cancer cells in the apheresis product: Potential clinical significance. In: Dicke K, Spitzer, Zander A, eds Autologous Marrow Transplantation: Proceedings of the Fourth International Symposium Houston: University of Texas M.D. Anderson Hospital and Tumor Institute at Houston. 4: 295–303, 1997
Vredenburg JJ, Tyer C, DeSombre K, Broadwater G, Berry D: The detection and significance of tumor cell contamination of the bone marrow. In: Dicke K, Spitzer, Zander A, eds Autologous Marrow Transplantation: Proceedings of the Fourth International Symposium. Houston: University of Texas M.D. Anderson Hospital and Tumor Institute at Houston, 4: 485–91, 1997
Moreb J, Cooper B, Holland K, Elliot C, Lazarus HM, Moss TJ: The prognostic value of immunocytochemical (IHC) analysis on bone marrow (BM) taken from patients with stage II/III breast cancer undergoing autologous transplant (ABMT) therapy. Blood 90:Suppl 1, 383a, 1997
Brugger W, Bross KJ, Glatt M, Weber F, Mertelsmann R, Kanz L: Mobilization of tumor cells and hematopoietic progenitor cells into peripheral blood of patients with solid tumors. Blood 83: 636–40, 1994
Abboud CN, Liesveld JL, Belanger TJ, Haug JS, Stamm C, Reykdal S, Rosell, Moss TJ, DikPersio JF: Progenitor kinetics after cytoxan G-, GM-, G + GM-CSF, or PIXY321 mobilization. Blood 90:Suppl 1, 98a, 1997
Brandt B, Roetger A, Heidi S, Jackisch C, Lelle RJ, Assman G, Zanker KS: Isolation of blood-borne epithelium-derived c-erb-2 oncoprotein positive clustered cells from the peripheral blood of breast cancer patients. Int J Cancer 76: 824–28, 1998
Umiel T, Pecora AL, Lazarus H, Kennedy MJ, Meagher R, Pecora AL, Rosenfeld C, Herzig R, Umiel T, Copelan E: Breast cancer contamination in peripheral blood stem cell (PBSC) collections associated with bone marrow disease and type of mobilization regimen. Blood 88:10 Suppl 1, 408, 1996.
Moss TJ, Ross AA: The risk of tumor cell contamination in peripheral blood stem cell collections. J Hematoth 1: 225–32, 1992
Moss TJ, Cairo MS, Bostrom B, Kletzel M, Kreissman S, Sender L, Cooper B, Lazarus HM, Kennedy MJ, Meagher R, Herzig RM, Ross AA: Using bone marrow (BM) immunocytochemical (ICC) analysis to determine optimal timing of peripheral blood stem cell (PBSC) harvest for patients with breast cancer and neuroblastoma. Blood 84:10 Suppl 1: 1401, 1994
Ross AA, Cooper BW, Lazarus HM, Moss TJ, Sweet DS, Winter C, Louie K, Madej P, Schneiderman E, Kahn DG, Warner NE: Different rates of detection of breast cancer cells in peripheral blood stem cell collections in single vs. multiple specimens. Proc Amer Soc Clin Oncol 13: 64, 1994
Kahn DG, Prilutskaya M, Cooper B, Kennedy MJ, Meagher R, Pecora AL, Preti RA, Mreb J, Wingard J, Rosenfeld C, Herzig R, Glassco JE, Umiel T, Copelan E, Lazarus HM, Moss TJ: The relationship between the incidence of tumor contamination and number of pheresis for stage IV breast cancer. Blood 90:Suppl 1, 565a, 1997
Moss TJ, Cooper B, Kennedy MJ, Meagher R, Pecora AL, Rosenfeld C, Herzig R, Umiel T, Copelan E, Lazarus HM: The prognostic value of immunocytochemical (IHC) analysis on bone marrow (BM) and stem cell products (PBSC) taken from patients with stage IV breast cancer undergoing autologous transplant therapy. Proc Amer Soc of Clin Oncol 16: 90a, 1997
Moss TJ, Umiel T, Herzig R, Meagher R, Cooper B, Kennedy MJ, Preti RA, Pecora AL, Copelan E, Lazarus HM, Rosenfeld C: The presence of clonogenic breast cancer cells in peripheral blood stem cell (PBSC) products correlates with an extremely poor prognosis for patients with stage IV disease. Blood 90:Suppl 1, 405b, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moss, T.J. Clinical Relevance of Minimal Residual Cancer in Patients with Solid Malignancies. Cancer Metastasis Rev 18, 91–100 (1999). https://doi.org/10.1023/A:1006264420822
Issue Date:
DOI: https://doi.org/10.1023/A:1006264420822