Abstract
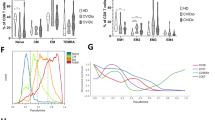
We present the case of a 28-year-old Caucasian female with common variable immunodeficiency (CVID) since age 5 who had a long history of hospitalizations for unexplained fevers and pulmonary infiltrates. The patient developed mild lymphocytosis 7 months prior to our evaluation. Flow cytometry of peripheral blood revealed an expansion of γδ T lymphocytes, mild CD4 T lymphocytopenia, and a reduced CD4/CD8 ratio (0.2). Two subpopulations of γδ T lymphocytes were found (CD3+/CD4−/CD8+, 47%; CD3+/CD4−/CD8−, 53%), the vast majority of which expressed V-δ1. An infectious cause for the patient's γδ T lymphocytosis could not be found. The sputum was chronically colonized with Staphylococcus aureus, and the organism produced TSST-1 in vitro. A bronchoalveolar lavage (BAL) revealed marked lymphocytosis, but γδ T lymphocytes were not overrepresented in the BAL. Lymphocyte functional studies revealed poor proliferative responses to mitogens and staphylococcal superantigens and diminished cytokine production. V-δ1 T lymphocytes from the patient's blood were not expanded in vitro in response to staphylococcal superantigens. TCR gene rearrangement studies confirmed the presence of Jγ and Jβ1 clonal rearrangements accounting for only a small subpopulation of the γδ T lymphocytes. These studies were repeated 5 months later and were unchanged. A bone marrow biopsy was negative for leukemia. Hence, the cause of the patient's γδ T lymphocytosis could not be determined despite evaluation for underlying malignancy, occult infection, or superantigen-driven stimulation. The patient ultimately died of progressive respiratory insufficiency. The state of current knowledge regarding γδ T lymphocytosis, decreased production of αβ T lymphocytes, and a low CD4/CD8 ratio in association with CVID is discussed.
Similar content being viewed by others
REFERENCES
Morio T, Nagasawa M, Yata J: Gamma-delta T cells in patients with primary immunodeficiency syndrome: Their function and a possible role in the pathogenesis. Chem Immunol 53:102–120, 1992
Lust JA, Letendre L, Thibodeau SN: Marrow hypoplasia associated with a monoclonal CD8 large granular lymphocyte proliferation: Reversal with cyclophosphamide and prednisone. Am J Med 87:215–217, 1989
Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S: Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. Nature 309:757, 1984
Brenner MB, McLean J, Dialynas DP, Strominger JL, et al.: Identification of a putative second T-cell receptor. Nature 322:145–149, 1986
Parker CM, Veronika G, Hamid B, Porcelli SA, et al.: Evidence for extrathymic changes in the T cell receptor g/d repertoire. J Exp Med 171:1597–1611, 1990
Haas W, Pereira P, Tonegawa S: Gamma/delta cells. Annu Rev Immunol 11:637–685, 1993
Kabelitz D: Function and specificity of human g/d-positive T cells. Crit Rev Immunol 1l(5):281–303, 1992
Scott CS, Richards SJ, Roberts BE: Patterns of membrane TCRab and TCR gd chain expression by normal blood CD4+ CD8−, CD4 CD8+, CD4− CD8drm− and CD4− CD8 lymphocytes. Immunology 70:351–364, 1990
Kabelitz D: Human gd T lymphocytes. Int Arch Allergy Immunol 102:1–9, 1993
Brunangelo F, Flenghi L, Piled S, Pelicci P, et al.: Distribution of T cells bearing different forms of the T cell receptor g/d in normal and pathological human tissues. J Immunol 143:2480–2488, 1989
Saiki O, Ralph P, Cunningham-Rundles C, Good R: Three distinct stages of B-cell defects in common varied immunodeficiency. Proc Natl Acad Sci USA 79:6008–6012, 1982
Farrant J, Bryant A, Almandoz F, Spickett G, Evans SW, Webster ADB: B cell function in acquired common variable hypogammaglobulinemia: Proliferative responses to lymphokines. Clin Immunol Immunopathol 51:196–204, 1989
Wright J, Wagner D, Blaese M, Hagengruber C, Waldmann T, Fleisher T: Characterization of common variable immunodeficiency: Identification of a subset of patients with distinctive immunophenotypic and clinical features. Blood 76:2046–2051, 1990
Loughran T: Clonal diseases of large granular lymphocytes. Blood 82:1–13, 1993
Foroni L, Laffan T, Boehm TH, Rabbitts D, et al.: Rearrangement of the T-cell receptor d genes in human T-cell leukemias. Blood 73:559–565, 1989
Foroni L, Matutes J, Foldi R, Morilla R, et al.: T-cell leukemia with rearrangement of the g but not b T-cell receptor. Blood 71:356–362, 1988
Catovsky D, Matutes E: Leukemias of mature T cells. In: Neoplastic Hematopathology, D Knowles (ed). Baltimore, Williams & Wilkins, 1992, pp 1267–1294.
Sklar J: Leukemias of mature T cells. In Neoplastic Hematopathology, D Knowles (ed). Baltimore, Williams & Wilkins, 1992, pp 1275–1276
Popa V: Lymphocytic interstitial pneumonitis in association with common variable immunodeficiency. Ann Allergy 60:203–206, 1988
Forrester J, Newman L, Wang Y, King T, Kotzin B: Clonal expansion of lung Vdl+ T cells in pulmonary sarcoidosis. J Clin Invest 91:292–300, 1993
Rust CJJ, Koning F: gd T cell reactivity towards bacterial superantigens. Semin Immunol 5:41–46, 1993
Paoli PD, Gennari D, Martelli P, Cavarzerani V, et al.: gd T cell receptor-bearing lymphocytes during Epstein-Barr virus infection. J Infect Dis 161:1013–1016, 1990
Autran B, Triebel F, Katlama C, Rozenbaum W, et al.: T cell receptor g/d+ lymphocyte subsets during HIV infection. Clin Exp Immunol 75:206–210, 1989
Born W, Harshan K, Modlin R, O'Brien R: The role of gd T lymphocytes in infection. Curr Opin Immunol 3:455–459, 1991
Kabelitz D, Bender A, Prospero T, Wesselborg S, Janssen O, Pechold K: The primary response of human gd+ T cells to Mycobacterium tuberculosis is restricted to Vg9-bearing cells. J Exp Med 173:1331–1338, 1991
Morio T, Masayuki N, Shigeaki N, Hiroji O, Yata J: Phenotypic profile and functions of T cell receptor-gd-bearing cells from patients with primary immunodeficiency syndrome. J Immunol 144:1270–1275, 1990
Bonneville M, Ishida I, Mombaerts P, Katsuki M, et al.: Blockage of ab T-cell development by TCR gd transgenes. Nature 342:931–934, 1989
Ishida I, Verbeek S, Bonneville M, Shigeyoshi I, et al.: T-cell receptor gd and g transgenic mice suggest a role of a g gene silencer in the generation of ab T cells. Proc Natl Acad Sci USA 87:3067–3071, 1990
Borowitz MJ, Guenther KL, Shults KE, Stelzer GT: Immunophenotyping of acute leukemia by flow cytometric analysis. Use of CD45 and right-angle light scatter to gate on leukemic blasts in three-color analysis. Am J Clin Pathol 100:534–540, 1993
BAL Cooperative Group Steering Committee: Bronchoalveolar lavage constituents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis 141(5, Pt 2):S169–S202, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Katial, R.K., Lieberman, M.M., Muehlbauer, S.L. et al. γδ T Lymphocytosis Associated with Common Variable Immunodeficiency1 . J Clin Immunol 17, 34–42 (1997). https://doi.org/10.1023/A:1027384311897
Issue Date:
DOI: https://doi.org/10.1023/A:1027384311897