Abstract
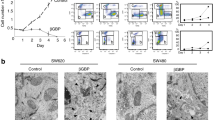
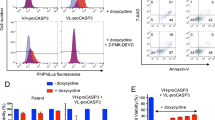
Tumor-associated antigens that can be recognized by the immune system include the MAGE-family, p53, MUC-1, HER2/neu and p21ras (refs. 1, 2, 3, 4, 5, 6). Despite their expression of these distinct antigens, tumor elimination by the immune system is often inefficient. Postulated mechanisms include insufficient expression of co-stimulatory or adhesion molecules by tumor cells, or defective processing and presentation of antigens on their cell surfaces7,8,9,10. Tumor cells may also evade immune attack by expressing CD95 (APO-1/Fas) ligand or other molecules that induce apoptosis in activated T cells11,12,13. Here we describe RCAS1 (receptor-binding cancer antigen expressed on SiSo cells), a membrane molecule expressed on human cancer cells. RCAS1 acts as a ligand for a putative receptor present on various human cell lines and normal peripheral lymphocytes such as T, B and NK cells. The receptor expression was enhanced by activation of the lymphocytes. RCAS1 inhibited the in vitro growth of receptor-expressing cells and induced apoptotic cell death. Given these results, tumor cells may evade immune surveillance by expression of RCAS1, which would suppress clonal expansion and induce apoptosis in RCAS1 receptor-positive immune cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
van de Bruggen, P. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643– 1647 (1991).
Melief, C.J. & Kast, W.M. T-cell immunotherapy of cancer. Res. Immunol. 142, 425–429 (1991).
Barnd, D.L., Lan, M.S., Metzgar, R.S. & Finn, O.J. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc. Natl. Acad. Sci. USA 86, 7159–7163 (1989).
Peiper, M. et al. The HER2/neu-derived peptide p654-662 is a tumor-associated antigen in human pancreatic cancer recognized by cytotoxic T lymphocytes. Eur. J. Immunol. 27, 1115–1123 (1997).
Van den Eynde, B.J. & Van der Bruggen, P. T cell defined tumor antigens. Curr. Opin. Immunol. 9, 684–693 (1997).
Sahin, U. Tureci, O. & Pfreundschuh, M. Serological identification of human tumor antigens. Curr. Opin. Immunol. 9, 709– 716 (1997).
Becker, J.C. et al. Tumor escape mechanisms from immunosurveillance: induction of unresponsiveness in a specific MHC-restricted CD4+ human T cell clone by the autologous MHC class II+ melanoma. Int. Immunol. 5, 1501–1508 (1993).
Runger, T.M., Klein, C.E., Becker, J.C. & Brocker, E.B. The role of genetic instability, adhesion, cell motility, and immune escape mechanisms in melanoma progression. Curr. Opin. Oncol. 6, 188–196 (1994).
Edward, M. Integrins and other adhesion molecules involved in melanocytic tumor progression. Curr. Opin. Oncol. 7, 185– 191 (1995).
Ferrone, S. & Marincola, F.M. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol. Today 16, 487– 494 (1995).
Susanne, S. et al. Lymphocyte apoptosis induced by CD95(APO-1/Fas) ligand-expressing tumor cells - A mechanism of immune evasion? Nature Med. 2, 1361–1366 (1996).
Claude, D.G. et al. Breast cancer-associated antigen, DF3/MUC1, induces apoptosis of activated human T cells. Nature Med. 2, 1367–1370 (1996).
Hahne, M. et al. Melanoma cell expression of Fas(Apo-1/CD95) ligand: Implications for tumor immune escape. Science 274, 1363 –1366 (1996).
Sonoda, K. et al. A novel tumor-associated antigen expressed in human uterine and ovarian carcinomas. Cancer 77, 1501– 1509 (1996).
Sonoda, K. et al. Establishment of a new human uterine cervical adenocarcinoma cell line, SiSo, and its reactivity to anti-cancer reagents. Int. J. Oncol. 6, 1099–1104 ( 1995).
Sonoda, K. et al. Tumor-associated antigen 22-1-1 expression in the uterine cervical squamous neoplasias. Clinical Cancer Research 4, 1517–1520 (1998).
K. Hofmann and W. Stoffel . TMBASE – A database of membrane spanning protein segments. Biol. Chem. Hoppe-Seyler 374, 166 (1993).
Gossen, M. et al. Transcriptional activation by tetracyclines in mammalian cells. Science 268, 1766–1769 (1995).
Henkart, P.A. ICE family proteases: mediators of all apoptotic cell death? Immunity , 4, 195–201 ( 1996).
Munker, R. et al. Expression of CD95(FAS) by gene transfer does not sensitize K562 to Fas-killing. Hematol. Cell Ther. 39, 75–78 (1997).
Owen-Schaub L.B. et al. Wild-type human p53 and a temperture-sensitive mutant Induce Fas/APO-1 Expression. Mol. Cell. Biol. 15, 3032–3040 (1995).
Whiteside, T.L. & Herberman, R.B. The role of natural killer cells in immune surveillance of cancer. Curr. Opin. Immunol. 7, 704–710 ( 1995).
Kaku T. et al. The prognostic significance of tumor-associated antigen 22-1-1 expression in adenocarcinoma of the uterine cervix. Clinical Cancer Research (in the press).
Watanabe T. et al. Isolation of estrogen-responsive genes with a CpG island library. Mol. Cell. Biol. 18, 442– 449 (1998).
Fukuda, T. et al. Restoration of surface IgM-mediated apoptosis in an anti-IgM-resistant variant of WEHI-231 lymphoma cells by HS-1, a protein-tyrosine kinase substrate. Proc. Natl. Acad. Sci. USA 92, 7302– 7306 (1995).
Acknowledgements
We thank P.D. Burrows and T.H. Bester for their critical reading and comments. We also thank H. Katsuta for his help. The work is supported by the Grant-in-Aid for Cancer Reserch from The Ministry of Education, Science and Culture of Japan (Grant number: 10153245).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakashima, M., Sonoda, K. & Watanabe, T. Inhibition of cell growth and induction of apoptotic cell death by the human tumor-associated antigen RCAS1. Nat Med 5, 938–942 (1999). https://doi.org/10.1038/11383
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/11383
This article is cited by
-
Diagnostic value of soluble receptor-binding cancer antigen expressed on SiSo cells and carcinoembryonic antigen in differentiating malignant from benign pleural effusion
Tumor Biology (2016)
-
Apoptotic function of tumor-associated antigen RCAS1 in oral squamous cell carcinoma
Journal of Translational Medicine (2014)
-
The role of RCAS1 as a biomarker in diagnosing CRC and monitoring tumor recurrence and metastasis
Tumor Biology (2014)
-
Serum-soluble receptor-binding cancer antigen expressed on SiSo cells as a clinical marker in lung cancer
Tumor Biology (2014)
-
Increased apoptosis and elevated Fas expression in circulating natural killer cells in gastric cancer patients
Gastric Cancer (2013)