Abstract
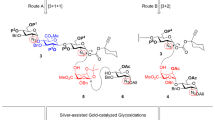
Unwanted side effects of pharmacologically active compounds can usually be eliminated by structural modifications. But the complex heterogeneous structure of the polysaccharide heparin1 has limited this approach to fragmentation, leading to slightly better-tolerated heparin preparations of low molecular mass2. Despite this improvement, heparin-induced thrombocytopaenia3 (HIT), related to an interaction with platelet factor 4 (PF4) and, to a lesser extent, haemorrhages4, remain significant side effects of heparinotherapy. Breakthroughs in oligosaccharide chemistry5 made possible the total synthesis of the pentasaccharide antithrombin-binding site of heparin6,7. This pentasaccharide represents a new family of potential antithrombotic drugs, devoid of thrombin inhibitory properties, and free of undesired interactions with blood and vessel components. To obtain more potent and well-tolerated antithrombotic drugs, we wished to synthesize heparin mimetics able to inhibit thrombin, that is, longer oligosaccharides. Like thrombin inhibition, undesired interactions are directly correlated to the charge and the size of the molecules8, so we had to design structures that were able to discriminate between thrombin and other proteins, particularly PF4. Here we describe the use of multistep converging synthesis to obtain sulphated oligosaccharides that meet these requirements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lane, D. A. & Lindahl, U. (eds) Heparin (Arnold, London, (1989).
Verstraete, M. Pharmacotherapeutic aspects of unfractionated and low molecular weight heparins. Drugs 40, 498–530 (1990).
Warkentin, T. E., Chong, B. H. & Greinacher, A. Heparin-induced thrombocytopenia: Towards consensus. Thromb.. Hemost. 79, 1– 7 (1998).
Thomas, D. P. Does low molecular weight heparin cause less bleeding? Thromb. Haemost. 78, 1422–1425 ( 1997).
Paulsen, H. Advances in selective chemical syntheses of complex oligosaccharides. Angew. Chem. Int. Ed. Engl. 21, 155– 173 (1982).
Choay, J. et al. Structure–activity relationship in heparin: a synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa Activity. Biochem. Biophys. Res. Commun. 116, 492 (1983).
van Boeckel, C. A. A. & Petitou, M. The unique antithrombin binding domain of heparin: a lead to new synthetic antithrombotics. Angew. Chem. Int. Ed. Engl. 32, 1671–1690 (1993).
Casu, B. Structure and biological activity of heparin. Adv. Carbohydr. Chem. Biochem. 43, 51–134 ( 1985).
Olson, S. T. & Björk, I. Regulation of thrombin activity by antithrombin and heparin. Semin. Thromb. Haemost. 20, 373–409 (1994).
Grootenhuis, P. D. J., Westerduin, P., Meuleman, D., Petitou, M. & van Boeckel, C. A. A. Rational design of synthetic heparin analogues with tailor-made coagulation factor inhibitory activity. Nature Struct. Biol. 2, 736– 739 (1995).
Laurent, T. C., Tengblad, A., Thunberg, L., Höök, M. & Lindahl, U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem. J. 175, 691–701 (1978).
Oosta, G. M., Gardner, W. T., Beeler, D. L. & Rosenberg, R. D. Multiple functional domains of the heparin molecule. Proc. Natl Acad. Sci. USA 78, 829–833 (1981).
Lane, D. A., Denton, J., Flynn, A. M., Thunberg, L. & Lindahl, U. Anticoagulant activities of heparin oligosaccharides and their neutralization by platelet factor 4. Biochem. J. 218, 725–732 (1984).
Danielsson, A., Raub, E., Lindahl, U. & Björk, I. Role of ternary complexes, in which heparin binds both antithrombin and proteinase, in the acceleration of the reactions between antithrombin and thrombin or factor Xa. J. Biol. Chem. 261, 15467–15473 (1986).
Basten, J. et al. Biologically active heparin-like fragments with a “non-glycosamino” glycan structure. Part 3: O -alkylated-O -sulphated pentasaccharides. Bioorg. Med. Chem. Lett. 2, 905– 910 (1992).
Petitou, M. et al. Synthesis and pharmacological properties of a close analogue of an antithrombotic pentasaccharide (SR 90107A/ORG 31540). J. Med. Chem. 40, 1600–1607 (1997).
Petitou, M. et al. First synthetic carbohydrates with the full anticoagulant properties of heparin. Angew. Chem. Int. Ed. Engl. 37, 3009–3014 (1998).
Herbert, J.-M. et al . Biochemical and pharmacological properties of SANORG 32701. Comparison with the “synthetic pentasaccharide” (SR 90107A/ORG 31540) and standard heparin. Circ. Res. 76, 590–600 (1996).
Umetsu, T. & Sanai, K. Effect of KC-6141, an anti-aggregating compound, on experimental thrombosis in rats. Thromb. Haemost. 39, 74–83 ( 1978).
Jin, J. et al. The anticoagulant activation of antithrombin by heparin. Proc. Natl Acad. Sci. USA 94, 14683–14688 (1997).
Westerduin, P. et al . Feasible synthesis and biological properties of six “non-glycosamino” glycan analogues of the antithrombin III binding heparin pentasaccharide. Bioorg. Med. Chem. 2, 1267– 1280 (1994).
Stubbs, M. T. & Bode, W. The clot thickens: clues provided by thrombin structure. Trends Biochem. Sci. 20, 23–28 (1995).
Sache, E. et al. Studies on a highly active anticoagulant fraction of high molecular weight isolated from porcine sodium heparin. Thromb. Res. 25, 443–458 (1982).
Olson, S. T., Halvorson, H. R. & Björk, I. Quantitative characterization of the thrombin-heparin interaction. Discrimination between specific and non-specific binding models. J. Biol. Chem. 266, 6342– 6352 (1991).
Maccarana, M. & Lindahl, U. Mode of interaction between platelet factor 4 and heparin. Glycobiology 3, 271 –277 (1993).
Atha, D. H., Lormeau, J. C., Petitou, M., Rosenberg, R. D. & Choay, J. Contribution of 3-O- and 6-O-sulfated glucosamine residues in the heparin-induced conformational change in antithrombin III. Biochemistry 26, 6454– 6461 (1987).
Sheridan, D., Carter, C. & Kelton, J. G. Adiagnostic test for heparin-induced-thrombocytopenia. Blood 67, 27–30 (1986).
Acknowledgements
This work is part of a collaborative project between N. V. Organon and Sanofi Recherche on antithrombotic oligosaccharides.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petitou, M., Hérault, JP., Bernat, A. et al. Synthesis of thrombin-inhibiting heparin mimetics without side effects . Nature 398, 417–422 (1999). https://doi.org/10.1038/18877
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/18877
This article is cited by
-
Interactions between Sclerostin and Glycosaminoglycans
Glycoconjugate Journal (2020)
-
By-Products of Heparin Production Provide a Diverse Source of Heparin-like and Heparan Sulfate Glycosaminoglycans
Scientific Reports (2019)
-
Bio-responsive polymer hydrogels homeostatically regulate blood coagulation
Nature Communications (2013)
-
Control of the heparosan N-deacetylation leads to an improved bioengineered heparin
Applied Microbiology and Biotechnology (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.