Abstract
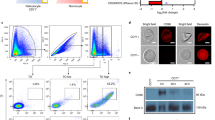
Several observations suggest that iron is essential for the development of malaria parasites1–6 but there is evidence that the parasites in erythrocytes do not obtain iron from haemoglobin. The total haemin level in parasitized erythrocytes does not vary during parasite development7, indicating that the iron-containing moiety of haemoglobin is not detectably metabolized. Although parasite proteases can degrade the protein part of haemoglobin in red cells8,9, no parasite enzymes that degrade haemin have been identified. In mammalian cells, haemin is degraded to carbon monoxide and bilirubin by the enzyme haeme oxygenase10. This enzyme has not been found in malaria parasites11. In fact haemin has been found to be toxic to parasite carbohydrate metabolism12. Thus, iron apparently cannot be liberated from haemin and instead is sequestered in infected red cells as haemozoin, the characteristic pigment associated with malarial infection13,14. If iron bound to transferrin is the source of ferric ions for malaria parasites within mature erythrocytes, then the parasite must synthesize its own transferrin receptor and localize it on the surface of the infected cell, because the receptors for transferrin are lost during erythrocyte maturation5,16. Our results here suggest that Plasmodium falciparum synthesizes its own transferrin receptors enabling it to take up iron from transferrin by receptor-mediated endocytosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Masawe, A. E. J., Muindi, D. M. & Suai, G. B. R. Lancet ii, 314–317 (1974).
Murray, M. S., Murray, A. B., Murray, M. B. & Murray, C. J. Br. med. J. 11, 1113–1115 (1979).
Raventos-Suarez, C., Pollack, S. & Nagel, R. L. Am. J. trop. Med. Hyg. 31, 919–922 (1982).
Harvey, P. W. J., Bell, R. G. & Nesheim, M. C. Infect. Immun. 50, 932–934 (1985).
Fritsch, G., Treumer, J., Spira, D. T. & Jung, A. Expl Parasitol. 60, 171–174 (1985).
Pollack, S. & Fleming, J. Br. J. Haematol. 58, 289–293 (1984).
Ball, E. G., McKee, R. W., Anfinsen, G. B., Cruz, W. O. & Geiman, Q. M. J. biol. Chem. 175, 547–571 (1948).
Moulder, J. W. & Evans, E. A. J. biol. Chem. 164, 145–157 (1940).
Levy, M. R., Siddiqui, W. A. & Chou, S. C. Nature 242, 546–549 (1974).
Landow, D., Callahan, Jr., E. W. & Schmid, R. J. clin. Invest. 49, 914–925 (1970).
Sherman, I. W. & Tanigoshi, L. Biochemistry of Parasites (ed. Slutzley, G. M.) 137–149 (Pergamon, Oxford, 1981).
Keilin, D. & Hartree, E. F. Biochem. J. 41, 403–406 (1947).
Rudzinska, M. A. & Trager, W. J. Biophys. biochem. Cytol. 6, 103–112 (1952).
Deegan, T. & Maegraith, B. G. Ann. trop. Med. Parasit 50, 194–211 (1956).
Van Bockxmeer, F. M. & Morgan, E. H. Biochim. biophys. Acta 584, 76–83 (1979).
Lodish, H. F. & Small, B. J. Cell. Biol. 65, 51–64 (1975).
Bleil, J. D. & Bretscher, M. S. EMBO J. 1, 351–355 (1982).
Van driel, I. R. Stearne, P. A., Greco, B., Simpson, R. J. & Goding, J. M. J. Immun. 133, 3220–3224 (1984).
McBride, J. S., Newbold, C. I. & Anand, R. J. exp. Med. 161, 160–180 (1985).
Nunez, M. T., Glass, J. & Cole, E. S. Biochem. biophys. Acta. 673, 137–146 (1981).
Tung, A. S., Shyr-te, S. & Nisonoff, A. J. Immun. 116, 676–681 (1976).
Rogers, J. & Jungery, M. (manuscript in preparation).
Leech, J. H., Barnwell, J. W., Miller, L. H. & Howard, R. J. J. exp. Med. 159, 1567–1575 (1984).
Schmidt-Ullrich, R., Wallach, D. F. H. & Lightholder, J. J. exp. Med. 150, 86–99 (1979).
Wallach, D. F. H. & Conley, M. J. molec. Med. 2, 119–136 (1977).
Trager, W. & Jensen, J. B. Science 193, 673–675 (1976).
Reese, R. T., Langreth, S. G. & Trager, W. Bull. WHO 57 (Suppl.), 53–61 (1979).
Bates, G. W. & Schlaback, M. R. J. biol. Chem. 250, 2177–2181 (1975).
Markelonis, G. J. et al. J. Cell Biol. 100, 8–17 (1985).
Mason, D. Y., Abdulaziz, Z., Falini, B. & Stein, H. Immunocytochemistry: Practical Applications in Pathology and Biology (eds Polak, J. M. & Van Noorden, S.) 113–118 (Wright-PSG, Bristol, 1983).
Laemmli, U. K. Nature 227, 680 (1970).
Burnette, W. N. Analyt. Biochem. 112, 195–203 (1981).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rodriguez, M., Jungery, M. A protein on Plasmodium falciparum-infected erythrocytes functions as a transferrin receptor. Nature 324, 388–391 (1986). https://doi.org/10.1038/324388a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/324388a0
This article is cited by
-
Identification of circulating biomarkers in sera of Plasmodium knowlesi-infected malaria patients – comparison against Plasmodium vivax infection
BMC Infectious Diseases (2015)
-
Direct access to serum macromolecules by intraerythrocytic malaria parasites
Nature (1991)
-
Structure and possible function ofPlasmodium falciparum proteins exported to the erythrocyte membrane
Parasitology Research (1991)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.