Abstract
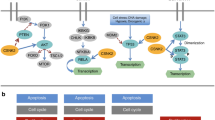
Members of the signal transducers and activators of transcription (STAT) pathway, which were originally identified as key components linking cytokine signals to transcriptional events in cells, have recently been demonstrated to have a major role in cancer. They are cytoplasmic proteins that form functional dimers with each other when activated by tyrosine phosphorylation. Activated STAT proteins translocate to the nucleus to regulate expression of genes by binding to specific elements within gene promoters. Constitutive activation of the STAT family members Stat3 and Stat5, and/or loss of Stat1 signaling, is found in a large group of diverse tumors. Increasing evidence demonstrates that STAT proteins can regulate many pathways important in oncogenesis including cell-cycle progression, apoptosis, tumor angiogenesis, tumor-cell invasion and metastasis, and tumor-cell evasion of the immune system. Based on these findings, a growing effort is underway to target STAT proteins directly and indirectly for cancer therapy. This review will highlight STAT signaling pathways, STAT target genes involved in cancer, evidence for STAT activation in human cancers, and therapeutic strategies to target STAT molecules for anticancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Darnell JE Jr (1997) STATs and gene regulation. Science 277: 1630–1635
Zhong Z et al. (1994) Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264: 95–98
Yu CL et al. (1995) Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269: 81–83
Yu H and Jove R (2004) The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 4: 97–105
Bowman T et al. (2000) STATs in oncogenesis. Oncogene 19: 2474–2488
Wen Z et al. (1995) Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82: 241–250
Bild AH et al. (2002) Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. EMBO J 21: 3255–3263
Zhang X et al. (1999) Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation. Mol Cell Biol 19: 7138–7146
Starr R et al. (1997) A family of cytokine-inducible inhibitors of signalling. Nature 387: 917–921
Chung CD et al. (1997) Specific inhibition of Stat3 signal transduction by PIAS3. Science 278: 1803–1805
Lufei C et al. (2003) GRIM-19, a death-regulatory gene product, suppresses Stat3 activity via functional interaction. EMBO J 22: 1325–1335
Reljic R et al. (2000) Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med 192: 1841–1848
He B et al. (2003) SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci U S A 100: 14133–14138
Shen Y et al. (2001) Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci U S A 98: 1543–1548
Catlett-Falcone R et al. (1999) Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10: 105–115
Epling-Burnette PK et al. (2001) Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest 107: 351–362
Real PJ et al. (2002) Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene 21: 7611–7618
Gritsko TT et al. Persistent activation of Stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res, in press
Diaz NM et al. (2005) Activation of Stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated Src and Survivin expression. Clin Cancer Res, in press
Niu G et al. (2001) Overexpression of a dominant-negative signal transducer and activator of transcription 3 variant in tumor cells leads to production of soluble factors that induce apoptosis and cell cycle arrest. Cancer Res 61: 3276–3280
Kiuchi N et al. (1999) STAT3 is required for the gp130-mediated full activation of the c-myc gene. J Exp Med 189: 63–73
Sinibaldi D et al. (2000) Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 19: 5419–5427
Niu G et al. (2002) Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21: 2000–2008
Wang T et al. (2004) Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 10: 48–54
Cheng F et al. (2003) A critical role for Stat3 signaling in immune tolerance. Immunity 19: 425–36
Xie TX et al. (2004) Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23: 3550–3560
Dauer DJ et al. (2005) Stat3 regulates genes common to both wound healing and cancer. Oncogene 24: 3397–3408
Xi S et al. (2003) Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res 63: 6763–6771
Kazansky AV et al. (2003) Activation of signal transducer and activator of transcription 5 is required for progression of autochthonous prostate cancer: evidence from the transgenic adenocarcinoma of the mouse prostate system. Cancer Res 63: 8757–8762
Huang M et al. (2002) Inhibition of Bcr-Abl kinase activity by PD180970 blocks constitutive activation of Stat5 and growth of CML cells. Oncogene 21: 8804–8816
Horita M et al. (2000) Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J Exp Med 191: 977–984
Nieborowska-Skorska M et al. (2002) Complementary functions of the antiapoptotic protein A1 and serine/threonine kinase pim-1 in the BCR/ABL-mediated leukemogenesis. Blood 99: 4531–4539
Li H et al. (2004) Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res 64: 4774–4782
Nevalainen MT et al. (2004) Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol 22: 2053–2060
Durbin JE et al. (1996) Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84: 443–450
Kaplan DH et al. (1998) Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 95: 7556–7561
Bromberg JF et al. (1996) Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A 93: 7673–7678
Chin YE et al. (1996) Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science 272: 719–722
Sun WH et al. (1998) Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 91: 570–576
Chen B et al. (2000) Inhibition of the interferon-gamma/signal transducers and activators of transcription (STAT) pathway by hypermethylation at a STAT-binding site in the p21WAF1 promoter region. Cancer Res 60: 3290–3298
Ramana CV et al. (2000) Regulation of c-myc expression by IFN-gamma through Stat1-dependent and -independent pathways. EMBO J 19: 263–272
Sironi JJ and Ouchi T (2003). STAT1-induced apoptosis is mediated by caspases 2, 3, and 7. J Biol Chem 279: 4066–4074
Stephanou A et al. (2000) Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and Bcl-x promoters. Cell Death Differ 7: 329–330
Thomas M et al. (2004) STAT1: a modulator of chemotherapy-induced apoptosis. Cancer Res 64: 8357–8364
Huang S (2002). Stat1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene 21: 2504–2512
Widschwendter A et al. (2002) Prognostic significance of signal transducer and activator of transcription 1 activation in breast cancer. Clin Cancer Res 8: 3065–3074
Mora LB et al. (2002) Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res 62: 6659–6666
Grandis JR et al. (2000) Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci U S A 97: 4227–4232
Song L et al. (2003) Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene 22: 4150–4165
Garcia R et al. (2001) Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20: 2499–2513
Gao H et al. (2004) Stat3 activation in acute lung injury. J Immunol 172: 7703–7712
Hokuto I et al. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest 113: 28–37
Sano S et al. (1999) Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J 18: 4657–6468
Kano A et al. (2003) Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J Exp Med 198: 1517–1525
Chan KS et al. (2004) Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest 114: 720–728
Greten FR et al. (2002) Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology 123: 2052–2063
Sanchez A et al. (2003) STAT-3 activity in chemically-induced hepatocellular carcinoma. Eur J Cancer 39: 2093–2098
Ren S et al. (2002) Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene 21: 4335–4339
Nikitakis NG et al. (2002) The nonsteroidal anti-inflammatory drug sulindac causes down-regulation of signal transducer and activator of transcription 3 in human oral squamous cell carcinoma cells. Cancer Res 62: 1004–1007
Turkson J (2004) STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets 8: 409–422
Blaskovich MA et al. (2003) Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res 63: 1270–1279
Turkson J et al. (2004) Inhibition of constitutive signal transducer and activator of transcription 3 activation by novel platinum complexes with potent anti-tumor activity. Mol Cancer Ther 3: 1–10
Turkson J et al. (2001) Phosphotyrosyl peptides block Stat3-mediated DNA-binding activity, gene regulation and cell transformation. J Biol Chem 276: 45443–45455
Ren Z et al. (2003) Identification of a high-affinity phosphopeptide inhibitor of stat3. Bioorg Med Chem Lett 13: 633–636
Nagel-Wolfrum K et al. (2004) The interaction of specific peptide aptamers with the DNA binding domain and the dimerization domain of the transcription factor Stat3 inhibits transactivation and induces apoptosis in tumor cells. Mol Cancer Res 2: 170–182
Jing N et al. (2003) Targeting Stat3 with G-quartet oligodeoxynucleotides in human cancer cells. DNA Cell Biol 22: 685–696
Leong PL et al. (2003) Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A 100: 4138–4143
Konnikova L et al. (2003) Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer 3: 23
Yin N et al. (2004) Molecular mechanisms involved in the growth stimulation of breast cancer cells by leptin. Cancer Res 64: 5870–5875
Giraud S et al. (2002) Functional interaction of STAT3 transcription factor with the coactivator NcoA/SRC1a. J Biol Chem 277: 8004–8011
Acknowledgements
The authors wish to thank members of their laboratories, colleagues who have contributed to the studies described, and acknowledge the important work of other investigators that could not be cited because of space limitations. Special thanks to Becky Alexander for administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- SRC-HOMOLOGY-2 (SH2)DOMAINS
-
Stretches of approximately 100 amino acids often found in signal transduction proteins, which confer binding to phosphorylated tyrosine residues within other signaling proteins
- GENE ASSOCIATED WITH RETINOID-INTERFERONINDUCED MORTALITY 19(GRIM 19)
-
The product of a cell-death regulatory gene induced by interferon-beta and retinoic acid
- BYSTANDER EFFECT
-
Tumor regression when a fraction of the tumor mass is genetically modified
Rights and permissions
About this article
Cite this article
Haura, E., Turkson, J. & Jove, R. Mechanisms of Disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Rev Clin Oncol 2, 315–324 (2005). https://doi.org/10.1038/ncponc0195
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0195
This article is cited by
-
STAT3 gene polymorphisms and susceptibility to breast cancer in the Moroccan population
Egyptian Journal of Medical Human Genetics (2023)
-
PROTAC’ing oncoproteins: targeted protein degradation for cancer therapy
Molecular Cancer (2023)
-
STAT signaling as a target for intervention: from cancer inflammation and angiogenesis to non-coding RNAs modulation
Molecular Biology Reports (2022)
-
Novel archetype in cancer therapeutics: exploring prospective of phytonanocarriers
3 Biotech (2022)
-
Oral microbiota and Helicobacter pylori in gastric carcinogenesis: what do we know and where next?
BMC Microbiology (2021)