Abstract
Amyloid is an abnormal extracellular fibrillar protein deposit in the tissues. In humans, more than 25 different proteins can adopt a fibrillar conformation in vivo that results in the pathognomonic tinctorial property of amyloid (that is, green birefringence when an affected tissue specimen is stained with Congo red dye and viewed by microscopy under cross-polarized light). Amyloid deposition is associated with disturbance of organ function and causes a wide variety of clinical syndromes that are classified according to the respective fibril protein precursor. Systemic amyloidosis, in which amyloid deposits are widespread and typically accumulate gradually, continues to be fatal and is responsible for about one in 1,500 deaths per year in the UK. Advances in our understanding of the pathogenesis of systemic amyloidosis have resulted in the identification of new therapeutic targets, and several drugs with novel mechanisms of action are currently under development. Meanwhile, an increased awareness of amyloidosis coupled with enhancements to existing diagnostic techniques and therapeutic strategies have already resulted in better outcomes for patients with the disease.
Key Points
-
More than 25 different human proteins can misfold to form amyloid fibrils in vivo; ∼15 of these misfolded proteins cause systemic amyloidosis, which is usually fatal
-
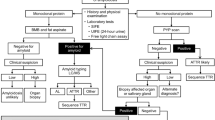
Diagnosis of amyloidosis relies on a high index of clinical suspicion and requires histological confirmation by staining of tissue specimens with Congo red dye
-
Identification of amyloid should prompt a series of investigations to identify the amyloid fibril protein and associated organ involvement and dysfunction
-
Therapies to enhance clearance of amyloid are in development; current treatment involves reducing the supply of the amyloid fibril precursor protein to slow or halt new amyloid formation
-
Amyloidotic organ dysfunction may gradually improve when amyloid formation is slowed or halted, and supporting organ function while waiting for clinical improvement is a crucial aspect of management
-
Advances in understanding of the molecular mechanisms involved in amyloid formation have led to the identification of several new therapeutic targets, and new therapeutic approaches are now in development
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sipe, J. D. et al. Amyloid fibril protein nomenclature: 2010 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid 17, 101–104 (2010).
Merlini, G. & Bellotti, V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 349, 583–596 (2003).
Obici, L., Raimondi, S., Lavatelli, F., Bellotti, V. & Merlini, G. Susceptibility to AA amyloidosis in rheumatic diseases: a critical overview. Arthritis Rheum. 61, 1435–1440 (2009).
Saraiva, M. J. Hereditary transthyretin amyloidosis: molecular basis and therapeutical strategies. Exp. Rev. Mol. Med. 4, 1–11 (2002).
Cohen, A. S. & Calkins, E. Electron microscopic observations on a fibrous component in amyloid of diverse origins. Nature 183, 1202–1203 (1959).
Eanes, E. D. & Glenner, G. G. X-ray diffraction studies on amyloid filaments. J. Histochem. Cytochem. 16, 673–677 (1968).
Chiti, F. & Dobson, C. M. Protein misfolding, functional amyloid, and human disease. Ann. Rev. Biochem. 75, 333–366 (2006).
Greenwald, J. & Riek, R. Biology of amyloid: structure, function, and regulation. Structure 18, 1244–1260 (2010).
Pepys, M. B. et al. Amyloid P component. A critical review. Amyloid 4, 274–295 (1997).
Bodin, K. et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 468, 93–97 (2010).
Li, J. P. et al. In vivo fragmentation of heparan sulfate by heparanase overexpression renders mice resistant to amyloid protein A amyloidosis. Proc. Natl Acad. Sci. USA 102, 6473–6477 (2005).
Motamedi-Shad, N., Monsellier, E., Torrassa, S., Relini, A. & Chiti, F. Kinetic analysis of amyloid formation in the presence of heparan sulfate: faster unfolding and change of pathway. J. Biol. Chem. 284, 29921–29934 (2009).
Ren, R. et al. Role of glycosaminoglycan sulfation in the formation of immunoglobulin light chain amyloid oligomers and fibrils. J. Biol. Chem. 285, 37672–37682 (2010).
Martin, D. J. & Ramirez-Alvarado, M. Glycosaminoglycans promote fibril formation by amyloidogenic immunoglobulin light chains through a transient interaction. Biophys. Chem. 158, 81–89 (2011).
Elimova, E., Kisilevsky, R. & Ancsin, J. B. Heparan sulfate promotes the aggregation of HDL-associated serum amyloid A: evidence for a proamyloidogenic histidine molecular switch. FASEB J. 23, 3436–3448 (2009).
Noborn, F. et al. Heparan sulfate/heparin promotes transthyretin fibrillization through selective binding to a basic motif in the protein. Proc. Natl Acad. Sci. USA 108, 5584–5589 (2011).
Ancsin, J. B. Amyloidogenesis: historical and modern observations point to heparan sulfate proteoglycans as a major culprit. Amyloid 10, 67–79 (2003).
Calero, M., Rostagno, A. & Ghiso, J. Search for amyloid-binding proteins by affinity chromatography. Methods Mol. Biol. 849, 213–223 (2012).
Hawkins, P. N. & Pepys, M. B. A primed state exists in vivo following histological regression of amyloidosis. Clin. Exp. Immunol. 81, 325–328 (1990).
Relini, A. et al. Collagen plays an active role in the aggregation of β2-microglobulin under physio-pathological conditions of dialysis-related amyloidosis. J. Biol. Chem. 281, 16521–16529 (2006).
Comenzo, R. L., Zhang, Y., Martinez, C., Osman, K. & Herrera, G. A. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V-L germ line gene use and clonal plasma cell burden. Blood 98, 714–720 (2001).
Perfetti, V. et al. Analysis of V λ-J λ expression in plasma cells from primary (AL) amyloidosis and normal bone marrow identifies 3r (λ III) as a new amyloid-associated germline gene segment. Blood 100, 948–953 (2002).
Abraham, R. S. et al. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood 101, 3801–3808 (2003).
Pepys, M. B. Amyloidosis. Annu. Rev. Med. 57, 223–241 (2006).
Sousa, M. M., Cardoso, I., Fernandes, R., Guimaraes, A. & Saraiva, M. J. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy—evidence for toxicity of nonfibrillar aggregates. Am. J. Pathol. 159, 1993–2000 (2001).
Andersson, K., Olofsson, A., Nielsen, E. H., Svehag, S. E. & Lundgren, E. Only amyloidogenic intermediates of transthyretin induce apoptosis. Biochem. Biophys. Res. Commun. 294, 309–314 (2002).
Hartley, D. M. et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19, 8876–8884 (1999).
Lambert, M. P. et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA 95, 6448–6453 (1998).
Walsh, D. M. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Shi, J. et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38α MAPK pathway. Proc. Natl Acad. Sci. USA 107, 4188–4193 (2010).
Liao, R. L. et al. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation 104, 1594–1597 (2001).
Brenner, D. A. et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circulation Res. 94, 1008–1010 (2004).
Silveira, J. R. et al. The most infectious prion protein particles. Nature 437, 257–261 (2005).
Palladini, G. et al. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood 107, 3854–3858 (2006).
Pinney, J. H. et al. Systemic amyloidosis in England: an epidemiological study. Br. J. Haematol. 161, 525–532 (2013).
Mesquita, M. et al. Renal biopsy findings in Belgium: a retrospective single center analysis. Acta Clin. Belg. 66, 104–109 (2011).
Tufveson, G. et al. The combined report on regular dialysis and transplantation in Europe. XIX 1988. Nephrol. Dial. Transplant. 4, 1–30 (1989).
Kyle, R. A. et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 346, 564–569 (2002).
Merlini, G. & Stone, M. J. Dangerous small B-cell clones. Blood 108, 2520–2530 (2006).
Obici, L., Perfetti, V., Palladini, G., Moratti, R. & Merlini, G. Clinical aspects of systemic amyloid diseases. Biochim. Biophys. Acta 1753, 11–22 (2005).
Witzig, T. E., Timm, M., Larson, D., Therneau, T. & Greipp, P. R. Measurement of apoptosis and proliferation of bone marrow plasma cells in patients with plasma cell proliferative disorders. Br. J. Haematol. 104, 131–137 (1999).
Rajkumar, S. V., Gertz, M. A. & Kyle, R. A. Primary systemic amyloidosis with delayed progression to multiple myeloma. Cancer 82, 1501–1505 (1998).
de Beer, F. C. et al. Serum amyloid A protein (SAA) concentration in inflammatory diseases and its relationship to the incidence of reactive systemic amyloidosis. Lancet 2, 231–234 (1982).
Schnitzer, T. J. & Ansell, B. M. Amyloidosis in juvenile chronic polyarthritis. Arthritis Rheum. 20, 245–252 (1977).
Myllykangas-Luosujärvi, R., Aho, K., Kautiainen, H. & Hakala, M. Amyloidosis in a nationwide series of 1666 subjects with rheumatoid arthritis who died during 1989 in Finland. Rheumatol. 38, 499–503 (1999).
Filipowicz-Sosnowska, A. M., Roztropowicz-Denisiewicz, K., Rosenthal, C. J. & Baum, J. The amyloidosis of juvenile rheumatoid arthritis—comparative studies in Polish and American children. I. Levels of serum SAA protein. Arthritis Rheum. 21, 699–703 (1978).
Svantesson, H., Akesson, A., Eberhardt, K. & Elborgh, R. Prognosis in juvenile rheumatoid arthritis with systemic onset. A follow-up study. Scand. J. Rheumatol. 12, 139–144 (1983).
Laiho, K., Tiitinen, S., Kaarela, K., Helin, H. & Isomaki, H. Secondary amyloidosis has decreased in patients with inflammatory joint disease in Finland. Clin. Rheumatol. 18, 122–123 (1999).
Immonen, K. et al. Decline in the incidence of renal failure due to amyloidosis associated with inflammatory rheumatic diseases. Amyloid 18 (Suppl. 1), 229–230 (2011).
Panizo, N., Rivera, F. & Lopez-Gomez, J. M. Decreasing incidence of AA amyloidosis in Spain. Eur. J. Clin. Invest. 43, 767–773 (2013).
Lachmann, H. J. et al. Natural history and outcome in systemic AA amyloidosis. N. Engl. J. Med. 356, 2361–2371 (2007).
Schwalbe, S. et al. β2-microglobulin associated amyloidosis: a vanishing complication of long-term hemodialysis? Kidney Int. 52, 1077–1083 (1997).
Benson, M. D., James, S., Scott, K., Liepnieks, J. J. & Kluve-Beckerman, B. Leukocyte chemotactic factor 2: a novel renal amyloid protein. Kidney Int. 74, 218–222 (2008).
Murphy, C. L. et al. Leukocyte chemotactic factor 2 (LECT2)-associated renal amyloidosis: a case series. Am. J. Kidney Dis. 56, 1100–1107 (2010).
Larsen, C. P., Walker, P. D., Weiss, D. T. & Solomon, A. Prevalence and morphology of leukocyte chemotactic factor 2-associated amyloid in renal biopsies. Kidney Int. 77, 816–819 (2010).
Kyle, R. A. & Gertz, M. A. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin. Hematol. 32, 45–59 (1995).
Pinney, J. H. et al. Outcome in renal AL amyloidosis following chemotherapy. J. Clin. Oncol. 29, 674–681 (2011).
Drueke, T. B. & Massy, Z. A. Beta2-microglobulin. Semin. Dial. 22, 378–380 (2009).
Nelson, S. R. et al. Imaging of haemodialysis-associated amyloidosis with 123I-serum amyloid P component. Lancet 338, 335–339 (1991).
Jimenez, R. E. et al. Development of gastrointestinal β2-microglobulin amyloidosis correlates with time on dialysis. Am. J. Surg. Pathol. 22, 729–735 (1998).
Sattianayagam, P. T. et al. Hereditary lysozyme amyloidosis—phenotypic heterogeneity and the role of solid organ transplantation. J. Intern. Med. 272, 36–44 (2012).
Obici, L. et al. Liver biopsy discloses a new apolipoprotein A-I hereditary amyloidosis in several unrelated Italian families. Gastroenterology 126, 1416–1422 (2004).
Gregorini, G. et al. Renal apolipoprotein A-I amyloidosis: a rare and usually ignored cause of hereditary tubulointerstitial nephritis. J. Am. Soc. Nephrol. 16, 3680–3686 (2005).
Gillmore, J. D. et al. Diagnosis, pathogenesis, treatment, and prognosis of hereditary fibrinogen A α-chain amyloidosis. J. Am. Soc. Nephrol. 20, 444–451 (2009).
Sethi, S. et al. Medullary amyloidosis associated with apolipoprotein A-IV deposition. Kidney Int. 81, 201–206 (2012).
Lobato, L. et al. End-stage renal disease in familial amyloidosis ATTR Val30Met: a definitive indication to combined liver-kidney transplantation. Transplant. Proc. 35, 1116–1120 (2003).
Valleix, S. et al. Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N. Engl. J. Med. 366, 2276–2283 (2012).
Rocken, C., Schwotzer, E. B., Linke, R. P. & Saeger, W. The classification of amyloid deposits in clinicopathological practice. Histopathology 29, 325–335 (1996).
van Gameren, I., Hazenberg, B. P., Bijzet, J. & van Rijswijk, M. H. Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 54, 2015–2021 (2006).
Fish, R. et al. The incidence of major hemorrhagic complications after renal biopsies in patients with monoclonal gammopathies. Clin. J. Am. Soc. Nephrol. 5, 1977–1980 (2010).
Arbustini, E. et al. Light and electron microscopy immunohistochemical characterization of amyloid deposits. Amyloid 4, 157–170 (1997).
Vrana, J. A. et al. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114, 4957–4959 (2009).
Lavatelli, F. et al. Amyloidogenic and associated proteins in systemic amyloidosis proteome of adipose tissue. Mol. Cell. Proteomics 7, 1570–1583 (2008).
Hawkins, P. N., Myers, M. J., Lavender, J. P. & Pepys, M. B. Diagnostic radionuclide imaging of amyloid: biological targeting by circulating human serum amyloid P component. Lancet 1, 1413–1418 (1988).
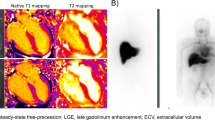
Falk, R. H. Diagnosis and management of the cardiac amyloidoses. Circulation 112, 2047–2060 (2005).
Westenberg, J. J. CMR for Assessment of Diastolic Function. Curr. Cardiovasc. Imaging Rep. 4, 149–158 (2011).
Hosch, W. et al. MR-relaxometry of myocardial tissue: significant elevation of T1 and T2 relaxation times in cardiac amyloidosis. Invest. Radiol. 42, 636–642 (2007).
Sparrow, P., Amirabadi, A., Sussman, M. S., Paul, N. & Merchant, N. Quantitative assessment of myocardial T2 relaxation times in cardiac amyloidosis. J. Magn. Reson. Imaging 30, 942–946 (2009).
Maceira, A. M. et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 111, 186–193 (2005).
Maceira, A. M., Prasad, S. K., Hawkins, P. N., Roughton, M. & Pennell, D. J. Cardiovascular magnetic resonance and prognosis in cardiac amyloidosis. J. Cardiovasc. Magn. Reson. 10, 54 (2008).
Rapezzi, C. et al. Usefulness and limitations of 99mTc-3, 3-diphosphono-1, 2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur. J. Nucl. Med. Mol. Imaging 38, 470–478 (2011).
Benson, M. D. Ostertag revisited: the inherited systemic amyloidoses without neuropathy. Amyloid 12, 75–87 (2005).
Merlini, G., Seldin, D. C. & Gertz, M. A. Amyloidosis: pathogenesis and new therapeutic options. J. Clin. Oncol. 29, 1924–1933 (2011).
Gillmore, J. D., Lovat, L. B., Persey, M. R., Pepys, M. B. & Hawkins, P. N. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet 358, 24–29 (2001).
Gertz, M. & Merlini, G. Definition of organ involvement and response to treatment in AL amyloidosis: an updated consensus opinion. Amyloid 17 (Suppl. 1), 48–49 (2010).
Comenzo, R. L. et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia 26, 2317–2325 (2012).
Gertz, M. A. et al. Effect of hematologic response on outcome of patients undergoing transplantation for primary amyloidosis: importance of achieving a complete response. Haematologica 92, 1415–1418 (2007).
Palladini, G. et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J. Clin. Oncol. 30, 4541–4549 (2012).
Lachmann, H. J. et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br. J. Haematol. 122, 78–84 (2003).
Wechalekar, A. D. et al. N-terminal fragment of brain natriuretic peptide (NT-ProBNP)—a new response criterion in AL amyloidosis. Amyloid 17 (Suppl. 1), 84–85 (2010).
Skinner, M. et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann. Intern. Med. 140, 85–93 (2004).
Cibeira, M. T. et al. Outcome of AL amyloidosis after high-dose melphalan and autologous stem cell transplantation: long-term results in a series of 421 patients. Blood 118, 4346–4352 (2011).
Gertz, M. A. et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 48, 557–561 (2013).
Gertz, M. A. et al. Clinical outcome of immunoglobulin light chain amyloidosis affecting the kidney. Nephrol. Dial. Transplant. 24, 3132–3137 (2009).
Dember, L. M. et al. Effect of dose-intensive intravenous melphalan and autologous blood stem-cell transplantation on AL amyloidosis-associated renal disease. Ann. Intern. Med. 134, 746–753 (2001).
Berglund, K., Thysell, H. & Keller, C. Results, principles and pitfalls in the management of renal AA-amyloidosis; a 10–21 year followup of 16 patients with rheumatic disease treated with alkylating cytostatics. J. Rheumatol. 20, 2051–2057 (1993).
Bergesio, F. et al. Renal involvement in systemic amyloidosis: an Italian collaborative study on survival and renal outcome. Nephrol. Dial. Transplant. 23, 941–951 (2008).
Tan, S. Y. et al. Long term effect of renal transplantation on dialysis-related amyloid deposits and symptomatology. Kidney Int. 50, 282–289 (1996).
Traut, M. et al. Increased binding of β2-microglobulin to blood cells in dialysis patients treated with high-flux dialyzers compared with low-flux membranes contributed to reduced β2-microglobulin concentrations. Results of a cross-over study. Blood Purif. 25, 432–440 (2007).
Gillmore, J. D. et al. Organ transplantation in hereditary apolipoprotein AI amyloidosis. Am. J. Transplant. 6, 2342–2347 (2006).
Coelho, T. et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology 79, 785–792 (2012).
Gertz, M. A., Kyle, R. A. & O'Fallon, W. M. Dialysis support of patients with primary systemic amyloidosis. A study of 211 patients. Arch. Intern. Med. 152, 2245–2250 (1992).
Moroni, G. et al. Chronic dialysis in patients with systemic amyloidosis: the experience in northern Italy. Clin. Nephrol. 38, 81–85 (1992).
Immonen, K. et al. No improvement in survival of patients with amyloidosis associated with inflammatory rheumatic diseases—data from the Finnish national registry for kidney diseases. J. Rheumatol. 35, 1334–1338 (2008).
Lachmann, H. J. et al. Survival on dialysis and outcome after renal transplantation in AA amyloidosis. Amyloid 17 (Suppl. 1), 73 (2010).
Sattianayagam, P. et al. Solid organ transplantation in AL amyloidosis. J. Am. Transplant. 10, 2124–2131 (2010).
Leung, N. et al. Living donor kidney and autologous stem cell transplantation for primary systemic amyloidosis (AL) with predominant renal involvement. Am. J. Transplant. 5, 1660–1670 (2005).
Emiroglu, R. et al. Effect of amyloidosis on long-term survival in kidney transplantation. Transplant. Proc. 37, 2967–2968 (2005).
Sherif, A. M. et al. Long-term outcome of live donor kidney transplantation for renal amyloidosis. Am. J. Kidney Dis. 42, 370–375 (2003).
Jacob, E. T., Bar-Nathan, N., Shapira, Z. & Gafni, J. Renal transplantation in the amyloidosis of familial Mediterranean fever. Experience of ten cases. Arch. Intern. Med. 139, 1135–1138 (1979).
Gillmore, J. D., Madhoo, S., Pepys, M. B. & Hawkins, P. N. Renal transplantation for amyloid end-stage renal failure - insights from serial serum amyloid P component scintigraphy. Nucl. Med. Commun. 21, 735–740 (2000).
Majumder, B. et al. “A case of cardiac amyloidosis with syncope”. Indian Heart J. 62, 171–172 (2010).
Palladini, G. et al. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood 103, 2936–2938 (2004).
Soni, A. & LeLorier, P. Sudden death in nondilated cardiomyopathies: pathophysiology and prevention. Curr. Heart Fail. Rep. 2, 118–123 (2005).
Kristen, A. V. et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 5, 235–240 (2008).
Lin, G., Dispenzieri, A., Kyle, R., Grogan, M. & Brady, P. A. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 24, 793–798 (2013).
Dubrey, S. W. et al. Long term results of heart transplantation in patients with amyloid heart disease. Heart 85, 202–207 (2001).
Maurer, M. S. et al. Cardiac transplantation using extended-donor criteria organs for systemic amyloidosis complicated by heart failure. Transplant. 83, 539–545 (2007).
Dey, B. R. et al. Cardiac transplantation followed by dose-intensive melphalan and autologous stem-cell transplantation for light chain amyloidosis and heart failure. Transplant. 90, 905–911 (2010).
Gillmore, J. D. et al. Sequential heart and autologous stem cell transplantation for systemic AL amyloidosis. Blood 107, 1227–1229 (2006).
Phipps, J. E. et al. Inhibition of pathologic immunoglobulin-free light chain production by small interfering RNA molecules. Exp. Hematol. 38, 1006–1013 (2010).
Benson, M. D. et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve 33, 609–618 (2006).
Kluve-Beckerman, B. et al. Antisense oligonucleotide suppression of serum amyloid A reduces amyloid deposition in mice with AA amyloidosis. Amyloid 18, 136–146 (2011).
US National Library of Medicine. ClinicalTrials.gov [online] (2013).
Sekijima, Y., Kelly, J. W. & Ikeda, S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr. Pharm. Des. 14, 3219–3230 (2008).
Dember, L. M. et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N. Engl. J. Med. 356, 2349–2360 (2007).
Pepys, M. B. et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature 417, 254–259 (2002).
Gillmore, J. D. et al. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br. J. Haematol. 148, 760–767 (2010).
Solomon, A., Weiss, D. T. & Wall, J. S. Immunotherapy in systemic primary (AL) amyloidosis using amyloid-reactive monoclonal antibodies. Cancer Biother. Radiopharm. 18, 853–860 (2003).
Merlini, G. et al. Interaction of the anthracycline 4′-iodo-4′-deoxydoxorubicin with amyloid fibrils: inhibition of amyloidogenesis. Proc. Natl Acad. Sci. USA 92, 2959–2963 (1995).
Cardoso, I. & Saraiva, M. J. Doxycycline disrupts transthyretin amyloid: evidence from studies in a FAP transgenic mice model. FASEB J. 20, 234–239 (2006).
Sattianayagam, P. T. et al. A prospective study of nutritional status in immunoglobulin light chain amyloidosis. Haematologica 98, 136–140 (2013).
Venner, C. P. et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood 119, 4387–4390 (2012).
Dispenzieri, A. et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 361, 1787–1789 (2003).
Lovat, L. B., Persey, M. R., Madhoo, S., Pepys, M. B. & Hawkins, P. N. The liver in systemic amyloidosis: insights from 123I serum amyloid P component scintigraphy in 484 patients. Gut 42, 727–734 (1998).
Acknowledgements
The work of the Centre for Amyloidosis and Acute Phase Proteins is supported by grants from the Medical Research Council (UK), The Wellcome Trust, the Wolfson Foundation, and NHS Research and Development Funds.
Author information
Authors and Affiliations
Contributions
J. D. Gillmore researched the data for the article. The authors contributed equally to writing the article, to discussions of the content, and to reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Gillmore, J., Hawkins, P. Pathophysiology and treatment of systemic amyloidosis. Nat Rev Nephrol 9, 574–586 (2013). https://doi.org/10.1038/nrneph.2013.171
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.171
This article is cited by
-
A case report of gastric amyloidosis due to multiple myeloma mimicking gastric cancer
BMC Gastroenterology (2020)
-
Clinical improvement of renal amyloidosis in a patient with systemic-onset juvenile idiopathic arthritis who received tocilizumab treatment: a case report and literature review
BMC Nephrology (2017)
-
Measurement of liver and spleen interstitial volume in patients with systemic amyloid light-chain amyloidosis using equilibrium contrast CT
Abdominal Radiology (2017)
-
D25V apolipoprotein C-III variant causes dominant hereditary systemic amyloidosis and confers cardiovascular protective lipoprotein profile
Nature Communications (2016)
-
Nationwide renal biopsy data in Lithuania 1994–2012
International Urology and Nephrology (2015)