Abstract
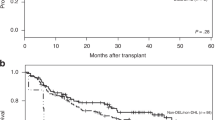
The prognostic value of the detection of peripheral blood (PB) and/or bone marrow (BM) involvement by polymerase chain reaction (PCR) amplification of rearranged immunoglobulin heavy chain (IgH) and immunoglobulin kappa light chain (Igκ) genes was evaluated in 155 patients with diffuse large B-cell lymphomas (DLBCL). Immunoglobulin gene rearrangements (IgR) were detected in 35/155 (23%) patients. The presence of IgR in PB/BM was related to clinical stage (CS I–III vs CS IV; P<0.001), histopathological detection of BM involvement (P<0.001), and the International Prognostic Index (P<0.001). IgR-positive cases had a significantly lower complete remission (CR) rate (18/35, 51%) than IgR-negative patients (85/120, 71%; P=0.042), and a significantly poorer overall survival (OAS) at 5 years (25 vs 66%; P<0.001). There was a significant difference in the estimated OAS at 5 years between patients with negative BM histology and negative PCR results (66%), patients with negative BM histology but positive IgR (37%), and patients with positive BM histology (12%). Our results indicate that molecular methods improve the accuracy of staging in patients with DLBCL and define a group of patients with normal bone marrow histology who have a significantly poorer OAS due to molecular detection of PB/BM involvement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med 1993; 328: 1002–1006.
Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med 1998; 339: 21–26.
A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329: 987–994.
Langerak AW, van Krieken JH, Wolvers-Tettero IL, Kerkhof E, Mulder AH, Vrints LW et al. The role of molecular analysis of immunoglobulin and T cell receptor gene rearrangements in the diagnosis of lymphoproliferative disorders. J Clin Pathol 2001; 54: 565–567.
Pongers-Willemse MJ, Seriu T, Stolz F, d'Aniello E, Gameiro P, Pisa P et al. Primers and protocols for standardized detection of minimal residual disease in acute lymphoblastic leukemia using immunoglobulin and T cell receptor gene rearrangements and TAL1 deletions as PCR targets: report of the BIOMED-1 CONCERTED ACTION: investigation of minimal residual disease in acute leukemia. Leukemia 1999; 13: 110–118.
van Dongen JJ, Seriu T, Panzer-Grumayer ER, Biondi A, Pongers-Willemse MJ, Corral L et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 1998; 352: 1731–1738.
Cave H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. European Organization for Research and Treatment of Cancer – Childhood Leukemia Cooperative Group. N Engl J Med 1998; 339: 591–598.
Kuppers R, Klein U, Hansmann ML, Rajewsky K . Cellular origin of human B-cell lymphomas. N Engl J Med 1999; 341: 1520–1529.
Stevenson F, Sahota S, Zhu D, Ottensmeier C, Chapman C, Oscier D et al. Insight into the origin and clonal history of B-cell tumors as revealed by analysis of immunoglobulin variable region genes. Immunol Rev 1998; 162: 247–259.
Derksen PW, Langerak AW, Kerkhof E, Wolvers-Tettero IL, Boor PP, Mulder AH et al. Comparison of different polymerase chain reaction-based approaches for clonality assessment of immunoglobulin heavy-chain gene rearrangements in B-cell neoplasia. Mod Pathol 1999; 12: 794–805.
Aubin J, Davi F, Nguyen-Salomon F, Leboeuf D, Debert C, Taher M et al. Description of a novel FR1 IgH PCR strategy and its comparison with three other strategies for the detection of clonality in B cell malignancies. Leukemia 1995; 9: 471–479.
Gong JZ, Zheng S, Chiarle R, Wolf-Peeters C, Palestro G, Frizzera G et al. Detection of immunoglobulin kappa light chain rearrangements by polymerase chain reaction. An improved method for detecting clonal B-cell lymphoproliferative disorders. Am J Pathol 1999; 155: 355–363.
Seriu T, Hansen-Hagge TE, Stark Y, Bartram CR . Immunoglobulin kappa gene rearrangements between the kappa deleting element and Jkappa recombination signal sequences in acute lymphoblastic leukemia and normal hematopoiesis. Leukemia 2000; 14: 671–674.
Hiorns LR, Nicholls J, Sloane JP, Horwich A, Ashley S, Brada M . Peripheral blood involvement in non-Hodgkin's lymphoma detected by clonal gene rearrangement as a biological prognostic marker. Br J Cancer 1994; 69: 347–351.
Horning SJ, Galili N, Cleary M, Sklar J . Detection of non-Hodgkin's lymphoma in the peripheral blood by analysis of antigen receptor gene rearrangements: results of a prospective study. Blood 1990; 75: 1139–1145.
Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML et al. A revised European–American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994; 84: 1361–1392.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting – Airlie House, Virginia, November, 1997. Hematol J 2000; 1: 53–66.
Armitage JO, Dick FR, Corder MP, Garneau SC, Platz CE, Slymen DJ . Predicting therapeutic outcome in patients with diffuse histiocytic lymphoma treated with cyclophosphamide, adriamycin, vincristine and prednisone (CHOP). Cancer 1982; 50: 1695–1702.
Fisher RI, DeVita Jr VT, Hubbard SM, Longo DL, Wesley R, Chabner BA et al. Diffuse aggressive lymphomas: increased survival after alternating flexible sequences of proMACE and MOPP chemotherapy. Ann Intern Med 1983; 98: 304–309.
Longo DL, DeVita Jr VT, Duffey PL, Wesley MN, Ihde DC, Hubbard SM et al. Superiority of ProMACE-CytaBOM over ProMACE-MOPP in the treatment of advanced diffuse aggressive lymphoma: results of a prospective randomized trial. J Clin Oncol 1991; 9: 25–38.
Pavlovsky S, Santarelli MT, Erazo A, Diaz Maqueo JC, Somoza N, Lluesma GM et al. Results of a randomized study of previously-untreated intermediate and high grade lymphoma using CHOP versus CNOP. Ann Oncol 1992; 3: 205–209.
O'Reilly SE, Connors JM, Howdle S, Hoskins P, Klasa R, Klimo P et al. In search of an optimal regimen for elderly patients with advanced-stage diffuse large-cell lymphoma: results of a phase II study of P/DOCE chemotherapy. J Clin Oncol 1993; 11: 2250–2257.
Szanto I, Fleischmann T, Eckhardt S . Treatment of resistant Hodgkin's disease with CCNU, etoposide and prednimustine (CEP). Oncology 1991; 48: 456–458.
Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol 1999; 17: 1244.
Sambrook J, Fritsch EF, Maniatis T . Molecular Cloning. A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press, 1989.
Frank TS, Svoboda-Newman SM, Hsi ED . Comparison of methods for extracting DNA from formalin-fixed paraffin sections for nonisotopic PCR. Diagn Mol Pathol 1996; 5: 220–224.
Sioutos N, Bagg A, Michaud GY, Irving SG, Hartmann DP, Siragy H et al. Polymerase chain reaction versus Southern blot hybridization. Detection of immunoglobulin heavy-chain gene rearrangements. Diagn Mol Pathol 1995; 4: 8–13.
Liang R, Chan V, Chan TK, Wong T, Chiu E, Lie A et al. Detection of immunoglobulin gene rearrangement in lymphoid malignancies of B-cell lineage by seminested polymerase chain reaction gene amplification. Am J Hematol 1993; 43: 24–28.
Trainor KJ, Brisco MJ, Wan JH, Neoh S, Grist S, Morley AA . Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood 1991; 78: 192–196.
Fodinger M, Winkler K, Mannhalter C, Chott A . Combined polymerase chain reaction approach for clonality detection in lymphoid neoplasms. Diagn Mol Pathol 1999; 8: 80–91.
Kwok S, Higuchi R . Avoiding false positives with PCR. Nature 1989; 339: 237–238.
Lo YM, Mehal WZ, Fleming KA . False-positive results and the polymerase chain reaction. Lancet 1988; 2: 679.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Macintyre EA, Delabesse E . Molecular approaches to the diagnosis and evaluation of lymphoid malignancies. Semin Hematol 1999; 36: 373–389.
Beishuizen A, de Bruijn MAC, Pongers-Willemse MJ, Verhoeven M-AJ, van Wering ER, Hählen K et al. Heterogeneity in junctional regions of immunoglobulin kappa deleting element rearrangements in B cell leukemias: a new molecular target for detection of minimal residual disease. Leukemia 1997; 11: 2200–2207.
Van der Velden VHJ, Willemse MJ, van der Schoot CE, Hählen K, van Wering ER, van Dongen JJM . Immunoglobulin kappa deleting element rearrangements in precursor-B acute lymphoblastic leukemia are stable targets for detection of minimal residual disease by real-time quantitative PCR. Leukemia 2002; 16: 928–936.
Szczepanski T, Willemse MJ, van Wering ER, van Weerden JF, Kamps WA, van Dongen JJM . Precursor-B-ALL with DH-JH gene rearrangements have an immature immunophenotype with a high frequency of oligoclonality and hyperdiploidy of chromosome 14. Leukemia 2001; 15: 1415–1423.
Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–511.
Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI et al. Lymphoma/Leukemia Molecular Profiling Project. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 1937–1947.
Acknowledgements
We are indebted to Susanne Hagmann for expert assistance with data management, and to Monika Heimbach and Helmut Sommer for excellent technical assistance. This study was supported by the FWF Grant P 13984-GEN and the Center of Molecular Medicine of the Austrian Academy of Sciences, and by Grant NB 9964 of the Austrian National Bank.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mitterbauer-Hohendanner, G., Mannhalter, C., Winkler, K. et al. Prognostic significance of molecular staging by PCR-amplification of immunoglobulin gene rearrangements in diffuse large B-cell lymphoma (DLBCL). Leukemia 18, 1102–1107 (2004). https://doi.org/10.1038/sj.leu.2403376
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403376
Keywords
This article is cited by
-
Prognostic markers for immunodeficiency-associated primary central nervous system lymphoma
Journal of Neuro-Oncology (2019)
-
Clinical significance of cytogenetic aberrations in bone marrow of patients with diffuse large B-cell lymphoma: prognostic significance and relevance to histologic involvement
Journal of Hematology & Oncology (2013)
-
Bone marrow involvement is predictive of infusion-related reaction during rituximab administration in patients with B cell lymphoma
Supportive Care in Cancer (2013)
-
Routine use of ancillary investigations in staging diffuse large B-cell lymphoma improves the International Prognostic Index (IPI)
Journal of Hematology & Oncology (2009)