Abstract
Background
The optimum management of patients whose needle core biopsy (NCB) results are of “uncertain malignant potential” (B3) or “suspicious for malignancy” (B4) is unclear. This study correlates B3 and B4 NCB findings with excision histology to determine associated rates of malignancy.
Methods
All NCBs categorized as B3 or B4 were identified from a series of 3729 NCBs. Results of biopsies were reported as normal/nondiagnostic (B1), benign (B2), uncertain malignant potential (B3), suspicious but not diagnostic of malignancy (B4), or malignant (B5) according to the B classification system. B3 lesions included atypical intraductal epithelial proliferations (AIEPs), lobular neoplasia, papillary lesions, radial scars, and potential phyllodes tumors. Histological concordance between NCB and excision specimen was analyzed.
Results
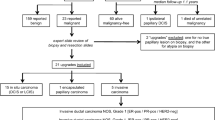
A total of 211 B3 lesions and 51 B4 lesions were identified during the study period. The open biopsy rate after a B3/B4 finding was 86% (n = 226). The overall rate of malignancy for B3 lesions after excision was 21%. The B3 lesion-specific rates of malignancy were 6% for radial scars, 14% for papillomas, 35% for AIEP, and 44% for lobular neoplasia. Of the patients with a B4 categorization, 90% (44 of 49) were diagnosed with carcinoma after surgery. Those that were “suspicious for ductal carcinoma-in-situ” and “suspicious for invasion” correlated accurately with excision findings in 81% and 89% of patients, respectively.
Conclusions
Management of lesions in the B3 categorization must be tailored to the patient because the specific lesion types are associated with highly variable rates of malignancy. A repeat biopsy or a therapeutic wide local excision should be undertaken in lesions with a B4 NCB categorization because such lesions are associated with a particularly high risk of malignancy at excision.
Similar content being viewed by others
References
Royal College of Pathologists. NHS Cancer Screening Programmes. Guidelines for Non Operative Diagnostic Procedures and Reporting In Breast Cancer Screening. Publication No. 50. NHSBSP, Sheffield, UK 2001. http://www.cancerscreening.nhs.uk/breastscreen/publications/nhsbps50.pdf
Dillon MF, Hill AD, Quinn CM, et al. The accuracy of ultrasound, stereotactic and clinical core biopsies in the diagnosis of breast cancer with an analysis of false negative cases. Ann Surg 2005; 242:701–7
Doyle JM, O’Doherty A, Coffrey L, et al. Can the radiologist accurately predict the adequacy of sampling when performing ultrasound-guided core biopsy of BI-RADS category 4 and 5 lesions detected on screening mammography? Clin Radiol 2005; 60:999–1005
Dennison G, Anand R, Maker SH, et al. A prospective study of the use of fine needle aspiration cytology and core biopsy in the diagnosis of breast cancer. Breast J 2003; 9:491–3
Liberman L, Dershaw DD, Rosen PP, et al. Stereotactic 14-gauge breast biopsy: how many core biopsy specimens are needed? Radiology 1994; 192:793–5
Brenner RJ, Fajardo L, Fisher PR, et al. Percutaneous core biopsy of the breast: effect of operator experience and number of samples on diagnostic accuracy. AJR Am J Roentgenol 1996; 166:341–6
Philpotts LE, Shaheen NA, Jain KS, et al. Uncommon high-risk lesions of the breast diagnosed at stereotactic core needle biopsy: clinical importance. Radiology 2000; 216:831–7
Jacobs TW, Connelly JL, Schnitt SJ. Non malignant lesions in breast core needles biopsies. To excise or not to excise? Am J Surg Pathol 2002; 26:1095–110
Reynolds HE. Core needle biopsy of challenging benign breast conditions: a comprehensive literature review. AJR Am J Roentgenol 2000; 174:1245–50
Frouge C, Tristant H, Guinebretiere JM, et al. Mammographic lesions suggestive of radial scars: microscopic findings in 40 cases. Radiology 1995; 195:623–5
Hassell P, Klein-Parker H, Worth A, et al. Radial sclerosing lesions of the breast: mammographic and pathologic correlation. Can Assoc Radiol J 1999; 50:370–5
Brodie C, Doherty A, Quinn C. Fourteen-gauge needle core biopsy of mammographically evident radial scars. Is excision necessary? Cancer 2004; 100:652–3
Sloane JP, Mayers MM. Carcinoma and atypical hyperplasia in radial scars and complex slerosing lesions: importance of lesion size and patient age. Histopathology 1993; 23:22–31
Douglas AG, Pace DP. Pathology of R4 spiculated lesions in the breast screening programme. Histopathology 1997; 30:214–20
Cawson JN, Malara F, Kavanagh A, et al. Fourteen-gauge needle core biopsy of mammographically evident radial scars. Is excision necessary? Cancer 2003; 97:345–51
Brenner RG, Jackman RJ, Parker SH, et al. Percutaneous core needle biopsy of radial scars of the breast. When is excision necessary? AJR Am J Roentgenol 2002; 179:1179–84
Carder PJ, Liston JC. Will the spectrum of lesions prompting a “B3” breast core biopsy increase the benign biopsy rate? J Clin Pathol 2003; 56:133–8
Carder PJ, Garvican J, Haigh I, Liston JC. Needle core biopsy can reliably distinguish between benign and malignant papillary lesions of the breast. Histopathology 2005; 46:320–7
Ivan D, Selinko V, Sahin AA, et al. Accuracy of core needle biopsy diagnosis in assessing papillary breast lesions: histological predictors of malignancy. Mod Pathol 2004; 17:165–71
Rosen EL, Bentley RC, Baker JA, et al. Imaging-guided core needle biopsy of papillary lesions of the breast. AJR Am J Roentgenol 2002; 179:1185–92
Liberman L, Bracero N, Vuolo MA, et al. Percutaneous large-core biopsy of papillary breast lesions. AJR Am J Roentgenol 1999; 172:331–7
Mercado CL, Harmele-Bena D, Singer C, et al. Papillary lesions of the breast: evaluation with stereotactic directional vacuum-assisted biopsy. Radiology 2001; 221:650–5
Rendels HE. Core needle biopsy of challenging benign breast conditions: a comprehensive literature review. AJR Am J Roentgenol 2000; 174:1245–50
Foster MC, Helvie MA, Gregory NE, et al. Lobular carcinoma in situ or atypical lobular hyperplasia at core needle biopsy: is excisional biopsy necessary? Radiology 2004; 231:813–9
Frykberg ER. Lobular carcinoma in situ of the breast. Breast J. 1999; 5:296–303
Arpino G, Allred DC, Mohsin SK, et al. Lobular neoplasia on core needle biopsy—clinical significance. Cancer 2004; 101:242–50
Page DL, Schuyler PA, Dupont WD, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. J Clin Pathol 2003; 361:125–9
Chuba PJ, Hamre MR, Yap J, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Pathol 2005; 23:534–41
Kopans DB. Lobular neoplasia on core-needle biopsy—clinical significance. Cancer 2004; 101:2902–3
Elsheikh TM, Silverman JF. Follow up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: a correlative study of 33 patients with review of the literature. Am J Surg Pathol 2005; 29:534–43
Komenaka IK, El-Tamer M, Pile-Spellman E, et al. Core needle biopsy as a diagnostic tool to differentiate phyllodes tumour from fibroadenoma. Arch Surg 2003; 138:987–90
Meyer JE, Smith DN, Lester SC, et al. Large-needle core biopsy: non malignant breast abnormalities evaluated with surgical excision or repeat biopsy. Radiology 1998; 206:717–20
Dillon MF, Quinn CM, McDermott EW, et al. Needle core biopsy in the diagnosis of phyllodes neoplasm. Surgery (in press)
Pinder SE, Ellis IO. Ductal carcinoma in situ (DCIS) and atypical ductal hyperplasia (ADH)—current definitions and classification. Breast Cancer Res 2003; 5:254–7
Dillon MF, Quinn CM, McDermott EW, et al. The diagnostic accuracy of core biopsy for ductal carcinoma in situ and its implications for surgical practice. J Clin Pathol 2006; 59:740–43
Elston CW, Sloane JP, Amendoeira I, et al. Causes of inconsistency in diagnosing and classifying intraductal proliferations of the breast. European Commission Working Group on Breast Screening Pathology. Eur J Cancer 2000; 36:1769–72
Sloane JP, Amendoeira I, Apostolikas N, et al. Consistency achieved by 23 European pathologists form 12 countries in diagnosing breast disease and reporting prognostic features of carcinomas. Virchows Archiv 1999; 434:3–10
Tavassoli FA, Devilee M, eds. World Health Organisation: Classification of Tumours: Pathology and Genetics. Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press, 2003
Viale G. Histopathology of primary breast cancer 2005. Breast 2005; 14:487–92
Van de Vijver MJ, Peterse H. The diagnosis and management of pre-invasive breast disease. Pathological diagnosis—problems with existing classifications. Breast Cancer Res 2003; 5:269–75
Verkooijen HM, Peeters PHM, Buskens E, et al. Diagnostic accuracy of large core needle biopsy for non palpable breast disease: a meta-analysis. Br J Can 2000; 82:1017–21
Rao A, Parker S, Ratzer E, et al. Atypical ductal hyperplasia of the breast diagnosed by 11-gauge directional vacuum-assisted biopsy. Am J Surg 2002; 184:534–7
Moore MM, Hargett CW 3rd, Hanks JB, et al. Association of breast cancer with the finding of atypical ductal hyperplasia at core breast biopsy. Ann Surg 1997; 225:726–31
Harvey JM, Sterrett GF, Frost FA. Atypical ductal hyperplasia and atypia of uncertain significance in core biopsies from mammographically detected lesions: correlation with excision diagnosis. Pathology 2002; 34:410–6
Ely KA, Carter BA, Jensen RA, et al. Core biopsy of the breast with atypical ductal hyperplasia: a probabilistic approach to reporting. Am J Surg Pathol 2001; 25:1017–21
Darling ML, Smith DN, Lester SC, et al. Atypical ductal hyperplasia and ductal carcimoma in situ as revealed by large-core needle breast biopsy: results of surgical excision. AJR Am J Roentgenol 2001; 177:250–1
Lee AH, Denley HE, Pinder SE, et al. Excision biopsy findings of patients with breast needle core biopsies reported as suspicious for malignancy (B4) or lesion of uncertain malignant potential (B3). Histopathology 2003; 42:331–6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dillon, M.F., McDermott, E.W., Hill, A.D. et al. Predictive Value of Breast Lesions of “Uncertain Malignant Potential” and “Suspicious for Malignancy” Determined by Needle Core Biopsy. Ann Surg Oncol 14, 704–711 (2007). https://doi.org/10.1245/s10434-006-9212-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9212-8