Article Text
Abstract
Background—It has been suggested that the subtyping of intestinal metaplasia in the stomach is useful in stratifying patients with regard to risk of developing gastric cancer.
Aim—To determine whether subtyping intestinal metaplasia provided useful information regarding the natural history of intestinal metaplasia.
Methods—The study used large cup gastric biopsy specimens from predetermined locations (gastric mapping). Follow up biopsies were obtained at one, two, and/or nine years. Biopsies with intestinal metaplasia were stained with high iron diamine/Alcian blue (HID/AB) to determine whether they expressed neutral mucins, sialomucins, or sulphomucins.
Results—Seventy nine patients with intestinal metaplasia were studied and characterised with regard to the most advanced subtype of intestinal metaplasia. The most severe type of intestinal metaplasia was type II in 33 patients and type III in 34 patients. Helicobacter pylori was cured in 67 patients. Follow up showed that changes in type of metaplasia (apparent regression or progression) occurred in both directions and were independent of H pylori status. For example, biopsy sites with “loss” of metaplasia at a follow up visit might have it “reappear” at a subsequent visit. During follow up, no patient developed gastric dysplasia or died from gastric cancer.
Conclusion—HID subtyping did not provide useful information to the clinician or the pathologist. The data are consistent with the notion that the pattern, extent, and severity of atrophy with/without intestinal metaplasia is a far more important predictor of increased cancer risk than intestinal metaplasia subtype.
- Helicobacter pylori
- intestinal metaplasia
- high iron diamine
- gastric cancer
Statistics from Altmetric.com
Intestinal-type gastric carcinoma is frequently accompanied by widespread intestinal metaplasia. Gastric cancer is believed to arise via a multistage process that includes chronic gastritis, gastric atrophy, usually with intestinal metaplasia, and finally dysplasia.1–8 It remains unclear whether intestinal metaplasia is a premalignant condition or a marker for increased risk of malignancy.2,9,10 The fact that intestinal metaplasia in the antrum is also found with duodenal ulcer disease, a condition associated with a low risk for the development of gastric cancer, suggests that other conditions or events are important in the pathogenesis of gastric cancer.11,12
The risk of developing gastric adenocarcinoma is highest in those with the most extensive atrophy associated with hypochlorhydria or achlorhydria.13–15 It has been suggested that there is a relation between cancer risk and the subtype of intestinal metaplasia, with the incidence of cancer being highest among patients with intestinal metaplasia subtype III.16–21 Confirmation of this hypothesis would suggest that typing of intestinal metaplasia could provide a simple approach to identify those at highest risk, and would allow resources to be directed to surveillance of that small group. Our study was designed to ask whether the results regarding the presence and type of intestinal metaplasia are reproducible or consistent on follow up of the same individual and whether type III metaplasia led to a high frequency of dysplasia.22
Materials and methods
PATIENTS
Mucosal biopsy specimens were obtained from patients who had previous upper gastrointestinal endoscopy with gastric mapping. Cases included patients with documented intestinal metaplasia who received surveillance endoscopy at six to 12 month intervals for up to nine years, and included patients in whom Helicobacter pylori infection had been treated successfully, in addition to treatment failures. Indications for upper gastrointestinal endoscopy included duodenal ulcer disease, gastric ulcer, non-ulcer dyspepsia, suspected mucosa associated lymphoid tissue lymphoma, volunteers, gastro-oesophageal reflux disease, or a previous diagnosis of intestinal metaplasia.
BIOPSY SPECIMENS
Each specimen was placed in a separate bottle of formalin and routinely processed. Serial sections were cut at 5 μm and stained with a triple stain, either the Genta stain23 or El-Zimaity triple stain.24,25 Each specimen was reviewed by one pathologist and scored using a visual analogue scale from 0 (absent/normal) to 5 (maximal intensity) for H pylori and intestinal metaplasia.26
HIGH IRON DIAMINE STAINING
Biopsies with intestinal metaplasia were stained with high iron diamine/Alcian blue (HID/AB) to identify neutral mucins, sialomucins, and sulphomucins. Briefly, slides were immersed in HID solution for 18 to 20 hours, at 23–25°C. Slides were then rinsed with deionised water and stained with 1% Alcian blue (pH 2.5) for two minutes.27 The evaluation of sialomucins and sulphomucins was done in sections without haematoxylin counterstain. Subtyping intestinal metaplasia was done according to the system used by Jass and Filipe17,22 as follows.
METAPLASIA SUBTYPING
Complete intestinal metaplasia
Type I: non-secretory absorptive cells and sialomucin secreting goblet cells.
Incomplete intestinal metaplasia
Type II: few absorptive cells, columnar cells secreting neutral and acid sialomucin, and goblet cells secreting mainly sialomucin but occasionally sulphomucin.
Type III: columnar cells secreting predominantly sulphomucin and goblet cells secreting sialomucin or sulphomucin.
ANALYSES
Patients were classified according to the most incomplete type of intestinal metaplasia. In addition, the change (apparent progression or regression) of intestinal metaplasia was analysed by site. Patients with more than five biopsies from the same sites obtained at multiple visits were further grouped with regard to the regions involved (antrum only v antrum and body). All scores were entered into a database and analysed using SigmaStat 2.03 (SPSS, Chicago, Illinois, USA). Fisher's exact test or, when appropriate, the χ2 test (both two tailed) were used for comparison of proportions. Significance of differences and relations were determined by p values of ⩽ 0.05.
Results
PATIENTS
Seventy nine patients, five women and 74 men (median age, 60; range, 32–80 years) were studied. Sixty seven patients were cured of H pylori infection; 12 remained infected at follow up. Follow ups were available at one year (n = 74), two years (n = 28), and nine years (n = 36). Three to 12 large cup (RADIAL JAW; MicroVasive, Watertown, Massachusetts, USA) gastric biopsy specimens were obtained from predetermined locations (fig 1). The minimal set was three, consisting of two antral (sites A3 and A4) and one corpus biopsy (site B6).
Gastric map showing biopsy sites. The letters A, B, and C refer to antrum, body, and cardia, respectively. The number after the letter (for example, A3) refers to specific sites.
PATIENTS NEGATIVE FOR h pylori at follow up
Sixty seven patients had a total of 1970 biopsies examined (average, 72/patient); 556 biopsies had intestinal metaplasia and were evaluated with HID/AB. Scoring was done according to the most advanced type of intestinal metaplasia. At the first visit, subtype II was present in 33 patients and subtype III was present in 34 patients (table 1).
Intestinal metaplasia subtype follow up in patients cured of Helicobacter pylori infection
One year follow up
Twenty three of the 33 patients initially classified with type II intestinal metaplasia had intestinal metaplasia type II at follow up; five had apparent regression to no intestinal metaplasia and five had undergone apparent progression to intestinal metaplasia type III.
Only 18 of the 33 patients initially classified as intestinal metaplasia type III had type III intestinal metaplasia at one year; 15 had apparently “regressed”, with eight having no metaplasia and seven having intestinal metaplasia type II.
Two year follow up
Twelve patients initially classified as type II intestinal metaplasia were evaluated two years after treatment. Type II intestinal metaplasia was present in seven; two had “progressed” to type III and three had no metaplasia found.
Thirteen of 34 patients initially classified as type III were evaluated after two years; no metaplasia was found in three and eight had regressed to intestinal metaplasia type II.
Nine year follow up
Five of the 33 patients initially classified as type II intestinal metaplasia died of unrelated diseases. Seven patients were evaluated at the nine year follow up: three had no metaplasia, three had intestinal metaplasia type II, and one had intestinal metaplasia type III.
Six of the 34 patients initially classified as type III intestinal metaplasia died of unrelated illnesses. Nine patients were evaluated and one had no metaplasia, five had intestinal metaplasia type II, and three had intestinal metaplasia type III.
PATIENTS WITH PERSISTENT h pylori infection
Twelve patients had persistent H pylori infection. A total of 250 biopsies were examined (average, 21/patient); 56 biopsies had intestinal metaplasia and were evaluated with HID/AB. Table 2 summarises the data. Six patients were initially classified as type III. At one year follow up, four continued to have type III intestinal metaplasia and two regressed to no metaplasia or type II intestinal metaplasia. At nine years, two of the six remaining patients had died of unrelated causes. Two were re-evaluated and one had no metaplasia and one had intestinal metaplasia type II. Six of these patients were initially classified as having intestinal metaplasia type II. Two were evaluated at one year follow up and one had no metaplasia and one had intestinal metaplasia type II. Five patients were evaluated after nine years and two continued to have intestinal metaplasia subtype II, one had apparent progression to type III, and two had no metaplasia.
Follow up of intestinal metaplasia subtypes in treatment failures
ANALYSIS BY BIOPSY SITE
At the first visit, 28 of 202 biopsies (14%) were type I, 109 (54%) were type II, and 65 (32%) were type III. Changes in type of metaplasia in both directions were seen independent of the presence or absence of H pylori. For example, in 11% of biopsy sites, metaplasia disappeared at one follow up visit but reappeared at a subsequent visit (table 3).
Changes in type of metaplasia in both directions were seen independent of the presence of Helicobacter pylori or cure of the infection
ANALYSIS ACCORDING TO REGION
To obtain a better representation of the distribution of intestinal metaplasia we performed a subgroup analysis restricted to 39 patients with more than five biopsies obtained at multiple visits, including 29 cured of the infection and 10 with persistent infection. The data were analysed according to the two working hypotheses: the presence of intestinal metaplasia in both the antrum and corpus has a worse prognosis than presence in the antrum only, and intestinal metaplasia with a visual analogue scale score of 5 is worse than that with a visual analogue scale of 1.
Patients cured of H pylori infection
After H pylori eradication, intestinal metaplasia status regressed in 18 patients, eight of whom initially had incomplete intestinal metaplasia type III. Apparent regression occurred irrespective of initial intestinal metaplasia subtype. Two patients had the same score before and after treatment with a 65 month follow up (intestinal metaplasia score was 4 and 5).
Patients with persistent H pylori infection
At presentation, all 10 patients had intestinal metaplasia in both the antrum and corpus and eight had intestinal metaplasia in both regions at subsequent visits. The two patients who did not appear to have intestinal metaplasia in subsequent visits had initial scores for intestinal metaplasia of 1.
Comparison of patients with intestinal metaplasia in both the antrum and corpus showed that the intestinal metaplasia status was unchanged in eight of 10 patients with persistent H pylori compared with 11 of 29 in whom the infection was cured (p = 0.03). When the comparison was done based on initial intestinal metaplasia score, no significant difference was observed between groups. For example, 13 patients who were subsequently cured of the infection had an initial intestinal metaplasia score ⩾ 3. On follow up, four showed apparent regression, eight showed no change, and one showed apparent progression. By comparison, in the untreated group, five had an initial intestinal metaplasia score ⩾ 3. No changes were seen in any of the patients (p = 0.27).
Discussion
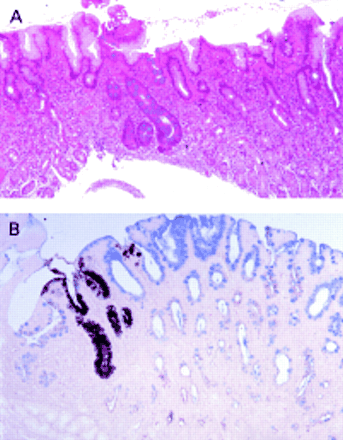
The association between gastric intestinal metaplasia and adenocarcinoma of the stomach is well known.1,28 Because the development of gastric carcinoma is slow and unpredictable, investigators have searched for premalignant markers of increased risk for gastric cancer. Intestinal metaplasia subtyped as III is often considered an ominous precursor lesion for the intestinal form of gastric cancer.16–19 Biopsy of the same areas of the stomach during follow up for up to nine years showed that neither the presence nor type of intestinal metaplasia was consistent, and that the type of metaplasia appeared to change in both directions. Areas of intestinal metaplasia (or a certain subtype) are generally small (fig 2), so that sampling error is probably responsible for the fact that an approximately equal number of studies have suggested that intestinal metaplasia regresses or does not regress after H pylori eradication.22,29,30–35 Sampling bias cannot be completely overcome by using targeted biopsies and jumbo forceps. Moreover, H pylori eradication did not decrease the likelihood of finding intestinal metaplasia in subsequent biopsies when comparisons were made between patients with the same initial score for intestinal metaplasia. Recent studies have shown that mucin protein expression is similar in types II and III intestinal metaplasia, so that examining the patterns of mucin protein expression cannot be used as a surrogate for the identification of type III intestinal metaplasia, and challenging the hypothesis that type II intestinal metaplasia consistently evolves into type III.36 Our study shows that, on follow up, typing intestinal metaplasia does not predict the pattern in subsequent biopsies or the outcome of the patient. Subsequent studies should probably examine both the type of intestinal metaplasia and mucin protein expression. The concept that type III intestinal metaplasia is predictive of a poor outcome has not been subjected to prospective trials and the natural history of the lesion is unknown. In our series, 18% of patients initially classified as intestinal metaplasia type III died of unrelated causes during follow up; none developed or died from gastric cancer. Only 20% of those with type III intestinal metaplasia had repeat biopsies showing type III intestinal metaplasia and none progressed to dysplasia or gastric carcinoma. These results appear to be different from those of Silva et al,22 who reported dysplasia on follow up of patients with type III intestinal metaplasia. However, they followed only eight patients for one year and three patients for five years. All those who developed dysplasia did so within the first year of the study, suggesting that it was probably present at the initial procedure.
In general, areas of intestinal metaplasia or a certain subtype are small and could easily go unsampled at follow up. (A) A small focus of intestinal metaplasia is seen in the middle of the section; El-Zimaity triple stain; original magnification, ×10. (B) A small focus of intestinal metaplasia type III among type II intestinal metaplasia; high iron diamine, original magnification, ×10.
In summary, it is not currently possible to make recommendations or prognoses based on single or multiple biopsies showing type III intestinal metaplasia.37 HID subtyping does not provide a reliable indicator of risk37–39 or provide prognostic value to the patient, the pathologist, or the endoscopist. Surveillance based solely on the presence of type III intestinal metaplasia appears unwarranted. The available data are consistent with the notion that the pattern, extent, and severity of atrophy with/without intestinal metaplasia are better predictors of risk than intestinal metaplasia subtype.40 The challenge to identify a reliable marker that further identifies a group as sufficiently high risk to justify endoscopic surveillance remains.