Article Text
Abstract
Aims The microtubule-associated protein 1 light chain 3 (LC3A) is an essential component of the autophagic vacuoles, forming a reliable marker of autophagic activity. In a previous study, the authors showed that LC3A immunohistochemistry renders three patterns of autophagic expression in breast carcinomas: diffuse cytoplasmic, perinuclear and ‘stone-like’ intracellular structures (SLS), each with a distinct prognostic relevance.
Methods Tumour tissues from 155 patients with stage IIA–III colorectal adenocarcinomas, treated with surgery alone, were assessed immunohistochemically for LC3A. Median values were used as cut-off points to separate groups into low and high autophagic activity. Associations with prognosis and with lactate dehydrogenase-5 (LDH5) were sought.
Results High SLS counts were associated with metastases and poor prognosis, while the prominence of the perinuclear pattern was linked to localised disease and good prognosis. The cytoplasmic pattern was irrelevant. Furthermore, patients with increased SLS numbers, but suppressed perinuclear expression, were associated with LDH5 overexpression and had an extremely poor prognosis (3-year survival 16.5%). The prognosis improved considerably when high SLS counts were accompanied by intense perinuclear expression (3-year survival 67%) and were optimal when SLS numbers dropped below median values, irrespective of perinuclear status (3-year survival 94–100%). Multivariate analysis showed that SLS and perinuclear patterns were independent predictors of death events.
Conclusions Perinuclear LC3A accumulation in colorectal tumour cells is a marker of good prognosis, presumably reflecting a basal autophagic activity. An abnormal or excessive autophagic response, as indicated by increased numbers of SLS, is linked to metastasis and poor prognosis.
- LC3A
- autophagy
- LDH5
- colorectal carcinomas
- cancer
- colorectal cancer
Statistics from Altmetric.com
Introduction
Hypoxia, acidity and nutrient deprivation are common in rapidly proliferating malignant tumours as a result of an inadequate vascular supply.1 Such tumours, being under the threat of cancer cell death, would respond to suboptimal oxygen and glucose distribution by activating the molecular cascade of hypoxia-inducible factors, and by upregulating anaerobic metabolic pathways.2 Autophagy is an additional adaptive mechanism by which cells recycle their own damaged organelles and proteins to generate metabolic fuel while, at the same time, preventing the accumulation of excess or defective cytoplasmic constituents.3 On the other hand, excessive autophagic activity may lead to cellular death.
Autophagy is characterised by the formation of double membrane vacuoles containing cytoplasmic constituents, the autophagosomes; these are fused with lysosomes to form the autolysosomes, which subsequently degrade the sequestered material. The role of autophagy in the growth and metastasis of primary human tumours remains poorly characterised. In a recent study, based on the expression of the light chain 3 (LC3A) protein, we showed that autophagy is upregulated in a variety of human carcinomas and in experimental tumours.4 Specific staining patterns were also recognised that may reflect a distinct functional status of the autophagic machinery.
LC3A, the microtubule-associated protein 1 light chain 3 (MAP1LC3A) (a homologue of yeast autophagy-related Atg protein 8), is an essential component of the autophagic vacuoles. LC3A exists in two forms, the LC3A-I (cytosolic) and the LC3A-II (membrane-bound).5 6 LC3A-II derives from a proLC3 30 kDa protein after cleavage by autophagin Atg4 to produce the active cytosolic form LC3A-I (18 kDa). This in turn is activated by Atg7 and Atg3, becoming a membrane-bound form, LC3-II. The latter binds tightly to preautophagosomal, autophagosomal and autolysosomal membranes forming a suitable marker of autophagic activity.
In this study, we assessed the patterns of LC3A expression in a series of colorectal adenocarcinomas treated with surgery alone. Our aim was to investigate the role of autophagy in the growth, metastasis and clinical behaviour of these common tumours.
Materials and methods
Patient-specimen characteristics
The material of this study comprised formalin-fixed, paraffin-embedded tissues from 155 consecutive patients with colorectal adenocarcinomas treated with surgery alone. It was collected from the archives of the Department of Cellular Pathology, John Radcliffe Hospital, Oxford, UK, and ethical approval was obtained from the appropriate institutional boards. The study was also approved by the Research Committee of the Democritus University of Thrace, Alexandroupolis, Greece. Eighty cases were stage IIA (T3-N0) and 75 were stage III (T3-N1,2) according to the TNM, AJCC/UICC staging system. Forty-nine of the 155 cases were tumours of rectal location. Sixty-six cases were female and 89 male. The median age of the patients was 69 years (range 37–87). The median follow-up period was 24 months (range 3–43 months).
LC3A assay methods
The purified rabbit polyclonal antibody MAP1LC3A (Abgent, San Diego, California), raised against a synthetic peptide at the C-terminal cleavage site of the human cleaved MAP1LC3A, was used for detecting autophagy. The immunogen sequence of the autophagy cleaved-LC3 antibody MAP1LC3A (AP1805a) at the C-terminal cleavage site of the human cleaved-LC3 (APG8a) is: DEDGFLYMVYASQETFG aa 104–120 (personal communication). The antibody is capable of detecting both the LC3-I and LC3-II forms, as confirmed by western blotting (data not shown). This particular antibody was selected above two other commercially available reagents, as showing an improved antigenic reactivity compared with those tested in parallel.
To establish the optimum concentration (titre) and incubation time for the primary MAP1LC3A (AP1805a) antibody, a series of colorectal adenocarcinomas were employed in a pilot study. The dilution giving the best contrast between the apparently positive malignant epithelial cells and the surrounding tumour stroma with minimal background staining was 1:20 after overnight incubation.
Tissue sections were cut at 3 μm and stained using a standard immunohistochemical technique. They were dewaxed and rehydrated in graded alcohol solutions. For heat-induced epitope retrieval, the sections were placed in citrate buffer (1:10 dilution, pH 7.2) and heated at 120°C for 3×5 min. Endogenous peroxidase activity was neutralised using Peroxidase Block for 5 min. The non-specific binding was blocked by preincubation with Protein Block for 5 min at room temperature (Novocastra Laboratories). Slides were then incubated overnight at 4°C with MAP1LC3A (AP1805a) primary antibody diluted 1:20 (Abgent, San Diego, California). The slides were washed with PBS (2×5 min) and then incubated with Post Primary Block (which enhances penetration of the subsequent polymer reagent) for 30 min at room temperature (Novocastra Laboratories). Thereafter, the sections were washed with PBS for 2×5 min and incubated with NovoLink polymer for 30 min at room temperature (Novocastra Laboratories). This recognises mouse and rabbit immunoglobulins and detects any tissue-bound primary antibody. After extensive washing with PBS (2×5 min), the colour reaction was developed in 3,3′-diaminobenzidine (DAB) for 5 min. The sections were then counterstained with haematoxylin, dehydrated and mounted.
Normal rabbit immunoglobulin-G was substituted for the primary antibody as a negative control. Staining with omission of the primary antibody was also performed as a negative control.
The assessment of autophagic activity using anti-LC3A antibodies in breast and other malignancies has been reported in a previous study of ours.4 Three distinct patterns were recognised: (a) diffuse cytoplasmic, (b) cytoplasmic/juxta-nuclear and (c) a ‘stone-like’ pattern—dense, rounded, amorphous structures typically enclosed within cytoplasmic vacuoles. These patterns were also recognised in HCT116 colon cancer spheroids and MDA231 breast cancer xenografts. Ultrastructural investigation with electron microscopy of tumour spheroids confirmed the presence of large, dense, rounded, amorphous material representing the ‘stone-like’ structures (SLS). Vacuoles containing masses of membranous debris within tightly fitting vacuoles in the cell cytoplasm were readily identified. Moreover, immunoblot analysis in MCF-7 human breast cancer cell lines confirmed induction of LC3A by anoxia and the endoplasmic reticulum stress agent Thapsigargin.
Briefly, the three patterns of LC3A activity were assessed as follows. The proportion of tumour cells expressing a diffuse cytoplasmic pattern per section was recorded at 100× magnification, and the median value of all cases studied was used to group tumours into low (<median) and high (≥median) cytoplasmic reactivity. The juxta/perinuclear pattern was evaluated in a similar way. The number of SLS were counted in all available fields of a section at 400× magnification and expressed as the mean of all counts. On the basis of the median value, the colorectal tumours in the series were subsequently divided into groups of low (<median) and high reactivity (≥median). The pathologists who performed the immunohistochemical assessment of LC3A were blinded to the patient, histopathological and outcome data.
LDH5 assay method
In order to assess the presence of a hypoxic and acidic environment, the sheep polycloncal ab9002 (Abcam, Cambridge, UK) raised against human LDH5 purified from human placenta was used for immunohistochemistry, as previously described.7 LDH5 catalyses the anaerobic transformation of pyruvate to lactate that is released into the extracellular milieu by monocarboxylate transporters and contributes to the acidification of the matrix. The scoring system for LDH5 has been previously reported.7 Briefly, LDH5 reactivity is both nuclear and cytoplasmic; tumours with nuclear reactivity in more than 10% of neoplastic cells and/or strong cytoplasmic reactivity above 50% were considered as highly expressing LDH5.
Assessment of vascular invasion and necrosis
The presence of tumour cells within well-defined vascular channels was recorded as positive for vascular invasion. Areas of necrosis extending over 10% of a tumour section were scored as extensive/positive, while the remaining cases were scored limited or negative. The assessment was performed on haematoxylin and eosin-stained sections.
Study design
This is a retrospective study based on archival material. The cases analysed represent a sequential series of patients according to the archival number given at the Department of Cellular Pathology, Oxford, UK, upon receipt of the surgical specimen. The selection of this specific material was performed to include patients who underwent surgery alone without chemotherapy of radiotherapy, thus simulating at a time when, in the lack of clinical evidence from properly designed randomised trials, these adjunctive therapies were not the golden standard. In this way, the analysis of data will reflect the actual impact of the tumour biology on the clinical outcome, without introducing into our model unpredictable differences in terms of chemo- or radiosensitivity of individual carcinomas. All patients in the series underwent surgery between 1990 and 1994, having a follow-up of at least 3 years after surgery. Patients with perioperative death and those with missing demographic or histopathological data were excluded from this study.
The end points of analysis were the association of LC3A expression with histopathological and immunohistochemical variables and its impact on the overall disease-specific survival. The overall statistical behaviour of the current series to these end-points has been previously tested and reported,7 providing significant differences for stage and immunohistochemical markers.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5.0 and the Instat 3.1 package (GraphPad Software, San Diego, California). LC3A scoring was handled as both a continuous and categorical variable. A Fisher exact test was used for testing relationships between categorical variables (contingency tables) as appropriate. Linear regression analysis was used to assess correlation with continuous variables. The Kaplan–Meier survival curves were used to assess the impact of various variables in the overall survival of patients. A Cox proportional hazard model was used to assess the effect of assessed parameters on death events. A p value of <0.05 was used for significance.
Results
Patterns of LC3A autophagic activity
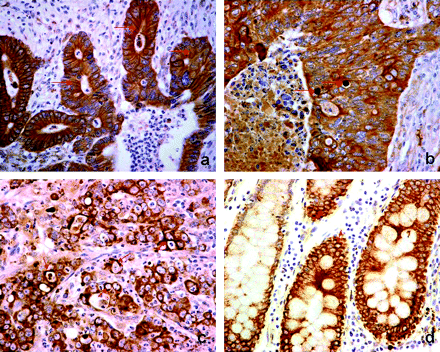
The three patterns of LC3A reactivity, that is diffuse cytoplasmic, cytoplasmic/juxta-nuclear, and the ‘stone-like’ pattern, are shown in figure 1. The median percentage of neoplastic cells with a diffuse cytoplasmic LC3A expression was 30% (range 0–90) per section, while that with perinuclear accumulation of LC3A was 10% (range 0–80). The median number of cancer cells with SLS was 2 (range 0–8) per optical field at 400× magnification. Using these median values, cases were grouped into low (<median) and high (≥median) reactivity categories.
Patterns of light chain 3 (LC3A) reactivity in colorectal adenocarcinomas and the adjacent ‘normal’ intestinal epithelium (magnification 400×). (A) Diffuse cytoplasmic pattern, as expressed by colonic tumour cells (arrows). (B) Stone-like structures (SLS) within autophagic vacuoles (arrows) of tumour cells. (C) Cytoplasmic/ perinuclear pattern of LC3A expression in colonic tumour cells (arrows). (D) ‘Normal’ colonic epithelium in the proximity of a tumour showing cytoplasmic and perinuclear expression.
Thus, a high autophagic reactivity in the form of a diffuse cytoplasmic pattern was noted in 82/155 cancer cases. Such cytoplasmic expression above median was associated with a similar increase in perinuclear (p=0.0002) and SLS expression (p=0.0001) (table 1). The perinuclear pattern was increased in 105/155 colorectal cancer cases, while the number of SLS was high in 103/155 cases.
Associations between light chain 3 (LC3) expression patterns in colorectal adenocarcinomas
Note that normal intestinal epithelium adjacent to colorectal tumours often showed a diffuse cytoplasmic and/or perinuclear expression of LC3A but consistently lacked SLS.
Association of LC3A with histopathological variables
The association of LC3A expression with various prognostic variables of colorectal tumours is shown in table 2. High SLS counts were associated significantly with the development of distant metastases (p<0.0001). By contrast, a prevalence of the perinuclear pattern was connected with a lack of metastases and localised disease (p=0.01). The same LC3A patterns were linked to tumour necrosis (p=0.04) and inversely with LDH5 (p=0.01). A trend for SLS to be linked to an advanced stage of disease and high-grade tumours did not reach statistical significance (p=0.09). The pattern of diffuse cytoplasmic staining was not linked with any variable.
Association of light chain 3 expression with histopathological variables in colorectal adenocarcinomas
The combined analysis of LC3A patterns (table 3) revealed interesting results, particularly with regard to the SLS and perinuclear patterns. Thus, the simultaneous presence of high SLS and low perinuclear expression was associated with vascular invasion (p=0.05), distant metastases (p<0.0008) and advanced stage of disease (p=0.001). Furthermore, the high SLS/low perinuclear combination was connected with overexpression of LDH5 (p=0.02). Suppression of any LC3A pattern was linked with lack of necrosis (p=0.0001).
Association of combined ‘stone-like’ structures/perinuclear light chain 3 expression patterns with histopathological variables in colorectal adenocarcinomas
Univariate survival analysis
Figure 2 shows the disease-specific overall survival Kaplan–Meier curves. High SLS counts were linked with poor prognosis (p<0.0001; HR 4.4, 95% CI 2.42 to 8.15); the 3-year survival was 52% in patients with high versus 96% in patients with low SLS. The opposite association was noted for the perinuclear LC3A pattern, where patients with values above the median had an improved survival over other patients (p<0.0001; HR 0.23; 95% CI 0.11 to 0.44); (3-year survival 77% vs 47%, respectively). The cytoplasmic staining was not linked to prognosis.
Kaplan–Meier survival curves in colorectal adenocarcinomas, according to the light chain 3A expression patterns: (A) ‘stone-like’ intracellular structures, (B) perinuclear and (C) diffuse cytoplasmic.
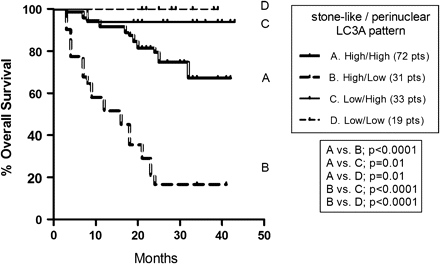
A combined analysis of SLS and perinuclear LC3A patterns is presented in figure 3. It was shown that patients with high SLS counts and low perinuclear expression had an extremely poor prognosis (3-year survival 16.5%). On the other hand, patients with low SLS counts had an excellent 3-year survival rate (94–100%), regardless of the perinuclear pattern. Patients with high SLS counts and high perinuclear expression had an intermediate prognosis (3-year survival 67%).
Kaplan–Meier survival curves with double stratification for ‘stone-like’ intracellular structures and perinuclear patterns in colorectal adenocarcinomas. LC3A, light chain 3.
Multivariate analysis
Multivariate analysis showed that SLS and perinuclear patterns, together with vascular invasion and stage, were strong independent predictors of death events (table 4).
Multivariate analysis of death events using variables significant at multivariate analysis
Discussion
The role of autophagy in maintaining cellular homeostasis and genome integrity has been amply emphasised,3 8 but its prognostic and predictive significance in human malignancies remains largely unexplored. Pirtoli, Li and Ding, each independently with their colleagues, connected the downregulation of Beclin1 (an essential protein for initiating autophagosome formation) with poor prognosis in high-grade gliomas, colonic and hepatocellular carcinomas,9–11 while Fujii et al associated a strong expression of LC3A with increased tumour size, extensive necrosis and poor survival in patients with pancreatic adenocarcinomas.12 Others suggested that an intensification of LC3 formation in cancer cells may be related to resistance to radiotherapy and the anti-HER2 monoclonal antibody trastuzumab.13 14
In a recent study, our group, investigating various epithelial tumours, demonstrated three distinct patterns of LC3A reactivity, namely the diffuse cytoplasmic, the perinuclear, and the SLS.4 The latter, being absent from normal intestinal, breast, endometrial and prostate epithelia, was considered as cancer cell restrictive and a hallmark of autophagic activity, at least in malignant epithelial cells. The SLS were readily made out as dense, rounded, amorphous, LC3A positive material, enclosed within cytoplasmic vacuoles; they occupied almost the whole cytoplasm, pushing the neoplastic nuclei to the cell periphery. Such structures, when in high numbers, were significantly linked with high-grade tumours and poor prognosis in breast carcinomas.4 The diffuse cytoplasmic pattern and the juxta/perinuclear LC3A accumulation was noted in both breast cancer cells and normal breast epithelia proximal to tumours, but only the perinuclear pattern was associated with steroid receptor expression and improved prognosis.
In the current study, increased SLS counts were detected in approximately two-thirds of the colorectal tumours examined, and so did independently the perinuclear expression pattern. By contrast, an intense diffuse cytoplasmic staining was directly linked with both the perinuclear and the stone-like patterns. It appears that a strong LC3A cytoplasmic staining reflects an intensified production of the soluble form of LC3A-I, and an early event/preparatory step in autophagosome formation. Once autophagosomes are formed, an important step in the autophagic process is the endocytic trafficking and delivery of autophagic cargo to the lysosome-rich perinuclear area.15 Thus, the perinuclear pattern of LC3A accumulation may be indicative of functional autophagic machinery.
In contrast, the SLS pattern, being a cancer-cell-restricted response, was thought to occur under conditions of extreme stress. Excessive autophagic stimulation may lead to an overload of degenerative cytoplasmic components and exhaustion of lysosomal enzymes. It follows accumulation of debris and, possibly, massive tumour cell destruction. This contention was supported by the strong link between SLS and lactate dehydrogenase 5 (LDH5), a marker of a hypoxic and acidic tumour environment, although a defective lysosomal function cannot be entirely excluded as being the cause of this SLS prominence. Indeed, the SLS appear to reflect an extreme autophagic response—an apparent consequence of a massive degradation of cellular components, which has been variously called ‘membranous whorls’ or ‘myelin figures.’16 17 This response may ultimately allow survival of cancer cells under stress and therefore associated with an aggressive phenotype. Stone-like patterns have been previously shown to correlate with high-grade breast carcinomas and, in this study, high-grade colorectal carcinomas expressed SLS twice as frequently, although this finding did not reach the level of statistical significance.
Combined analysis of perinuclear and SLS patterns showed a direct association of both patterns with necrosis, suggesting that hypoxia is a triggering event for the accumulation of LC3A. Indeed, as we have shown previously, hypoxia enhances the accumulation of LC3-A and -B forms in human breast cancer cell lines (MCF-7).4 Furthermore, a rapid induction of LC3-II levels by acute hypoxia has been confirmed by Chen et al.18 It appears that LC3 may be important in protecting tissues from hypoxic injury, given that RNAi knockdown of LC3 sensitised cells leads to hypoxic death.19
It is interesting that the prevalence of SLS, in the absence of an apparent perinuclear pattern, was significantly linked to nodal spread and lymphatic/vascular space invasion. The latter commonly results in distant metastases, a phenomenon which was manifested in the present study. In survival analysis, the high SLS counts were connected with extremely poor prognosis, especially when the perinuclear pattern was suppressed. This is probably because the presence of an intense perinuclear pattern, reflecting a basal rate of autophagic activity, seems to modify the aggressive qualities of SLS, allowing a better clinical course. Survival rates became, however, ideal only when SLS counts were below median. In a multivariate analysis, the pattern of SLS and that of perinuclear staining were independent and strong predictors of prognosis.
It is concluded that perinuclear accumulation of LC3A protein is a marker of good prognosis in colorectal adenocarcinomas, probably indicating a basal rate of functional autophagic machinery. An abnormal or excessive autophagic response, on the other hand, represented by high SLS counts, is linked with distant metastases and poor prognosis. Rapidly growing tumours survive the conditions of oxygen and nutrient exhaustion by turning to an overactivated autophagic state, characterised by increased numbers of SLS. Nonetheless, the possibility of a specific cancer cell abnormality affecting the autophagic/lysosomal machinery per se and influencing cancer growth and metastasis directly cannot be totally excluded. Additional clinicopathological studies are obviously needed in order to delineate the prognostic role of LC3A immunohistochemistry in colorectal oncology. These should also include patients treated with chemotherapy and/or radiotherapy for assessing the sensitivity of colorectal tumours to such agents in relation to the status of autophagic machinery.
Take-home messages
The microtubule-associated protein 1 LC3A is an essential component of the autophagic vacuoles, forming a reliable marker of autophagic activity.
Immunohistochemical detection of LC3A in colorectal carcinomas revealed three distinct patterns of autophagic expression: the diffuse cytoplasmic, (ii) the cytoplasmic/perinuclear and (iii) the SLS; the latter are dense, rounded, amorphous structures, of 5 μm average size, typically enclosed within cytoplasmic vacuoles.
High SLS counts, probably reflecting an excessive autophagic response, were associated with tumour hypoxia/acidity (high LDH5 expression), metastases and poor prognosis.
The diffuse cytoplasmic pattern does not seem to affect the outcome.
Prominence of the perinuclear pattern, indicative of a basal rate of autophagic function, was linked to localised disease and good prognosis.
References
Footnotes
Competing interests None.
Ethics approval The Oxfordshire Research Ethics Committee B approved our work no CO2.216. The study was also approved by the Research Committee of the Democritus University of Thrace, Alexandroupolis, Greece.
Provenance and peer review Not commissioned; externally peer reviewed.