Article Text
Abstract
Endogenous Cushing's syndrome is a rare endocrine disorder that incurs significant cardiovascular morbidity and mortality, due to glucocorticoid excess. It comprises adrenal (20%) and non-adrenal (80%) aetiologies. While the majority of cases are attributed to pituitary or ectopic corticotropin (ACTH) overproduction, primary cortisol-producing adrenal cortical lesions are increasingly recognised in the pathophysiology of Cushing's syndrome. Our understanding of this disease has progressed substantially over the past decade. Recently, important mechanisms underlying the pathogenesis of adrenal hypercortisolism have been elucidated with the discovery of mutations in cyclic AMP signalling (PRKACA, PRKAR1A, GNAS, PDE11A, PDE8B), armadillo repeat containing 5 gene (ARMC5) a putative tumour suppressor gene, aberrant G-protein-coupled receptors, and intra-adrenal secretion of ACTH. Accurate subtyping of Cushing's syndrome is crucial for treatment decision-making and requires a complete integration of clinical, biochemical, imaging and pathology findings. Pathological correlates in the adrenal glands include hyperplasia, adenoma and carcinoma. While the most common presentation is diffuse adrenocortical hyperplasia secondary to excess ACTH production, this entity is usually treated with pituitary or ectopic tumour resection. Therefore, when confronted with adrenalectomy specimens in the setting of Cushing's syndrome, surgical pathologists are most commonly exposed to adrenocortical adenomas, carcinomas and primary macronodular or micronodular hyperplasia. This review provides an update on the rapidly evolving knowledge of adrenal Cushing's syndrome and discusses the clinicopathological correlations of this important disease.
Statistics from Altmetric.com
Introduction
First described in 1932, Cushing's syndrome is a well known clinical entity that reflects chronic exposure of the body to excess glucocorticoids, from either endogenous or more commonly exogenous sources.1–5 Accurate epidemiological data on this topic is currently lacking.5 The reported incidence of endogenous cases ranges from 2 per million to 3 per million per year, with approximately 10% of cases occurring in children.1–6 Recognition of Cushing's syndrome is critical because the overall mortality from moderate-to-severe hypercortisolism is increased twofold due to macrovascular (myocardial infarction, stroke) and infectious complications.7 With modern-day treatments, mortality rates after normalisation of cortisol have been reported to be similar to that of an age-matched population.1–5 ,7
Endogenous Cushing's syndrome is classically divided into corticotropin (ACTH)-dependent and ACTH-independent hypercortisolism.1–12 The majority of cases of the ACTH-dependent subtype are caused by a pituitary ACTH-producing adenoma, also known as Cushing's disease (70% of endogenous Cushing's syndrome; table 1).1–4 Other cases are caused by ectopic ACTH secretion (10%) from a variety of neuroendocrine neoplasms including paraganglioma, phaeochromocytoma and neuroendocrine tumours of various sites (lung, thyroid, thymus, appendix and pancreas).1–6 Rare neuroendocrine tumours (<1%) can also present with Cushing's syndrome when they secrete corticotropin-releasing hormone (CRH).3 ,10 In contrast with the diverse aetiologies of ACTH-dependent Cushing's syndrome, ACTH-independent cases are attributed to primary cortisol-producing adrenocortical lesions (20%), which may arise sporadically or in the setting of rare genetic syndromes.8 ,11–13
Overview of endogenous Cushing
Accurate subtyping of Cushing's syndrome is essential for treatment decision-making and requires a complete integration of clinical, biochemical, imaging and pathology findings. Pathological correlates in the adrenal glands include hyperplasia, adenoma and carcinoma.11–13 While ACTH-dependent adrenal hyperplasia is the most common clinical manifestation of endogenous Cushing's syndrome, this entity rarely presents itself in surgical pathology because most cases are treated with transsphenoidal pituitary adenomectomy, ectopic ACTH-producing neuroendocrine tumour resection, radiation or medical therapy.2 ,3 ,14 ,15 Therefore, in surgical series of Cushing's syndrome, the most common adrenal pathologies relate to ACTH-independent hypercortisolism (adrenal Cushing's syndrome).16 Cortisol-producing adrenocortical adenoma and carcinoma account for, respectively, 55% and 35% of cases of adrenal Cushing's syndrome, while primary adrenocortical macronodular and micronodular hyperplasia have been attributed to the remainder 10% of cases (table 2).2 ,3 ,11–13
Clinical, biochemical and radiological features of adrenal Cushing
Clinical and biochemical aspects of Cushing's syndrome
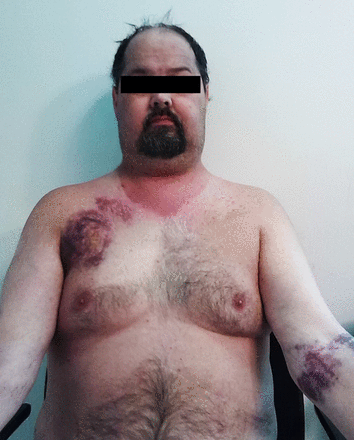
In clinical practice, Cushing's syndrome can present at all ages, with a female predominance.1–5 While the overt presentation is clinically unmistakable (central ‘truncal’ obesity, ‘moon facies’ and ‘buffalo humps’), milder cases are more difficult to diagnose, due to the wide spectrum of manifestations.1–4 None of its symptoms are pathognomonic and many of its features (obesity, diabetes, hypertension, fatigue, irritability, depression, decreased concentration and menstrual irregularity) are commonly seen in the general population.1–4 A clue to the presence of hypercortisolism is the concurrent development and increasing severity of multiple symptoms.1–4 Clinical signs that best discriminate Cushing's syndrome from obesity include easy bruising (figure 1), skin atrophy, unexplained osteoporosis, proximal muscle weakness, reddish purple striae and facial plethora.1 In children, weight gain with decreasing growth velocity has been described as a hallmark of Cushing's syndrome.1 ,6
Clinical features of Cushing's syndrome. The overt presentation is clinically unmistakable (central ‘truncal’ obesity, ‘moon facies’ and ‘buffalo humps’), milder cases are more difficult to diagnose, due to the wide spectrum of manifestations. None of its symptoms are pathognomonic and many of its features are commonly seen in the general population. Clinical signs that have been reported to best discriminate Cushing's syndrome from obesity include easy bruising, skin atrophy, unexplained osteoporosis, proximal muscle weakness, reddish purple striae and facial plethora.
Diagnostic considerations and pitfalls
When hypercortisolism is suspected based on clinical presentation, a thorough drug history (oral, injected, topical and inhaled corticosteroids) is necessary to rule out iatrogenic or exogenous aetiologies.1–4 Factitious Cushing's syndrome has also been reported in less than 1% of cases, and can be extremely difficult to recognise.17 Other pitfalls in the diagnosis of endogenous Cushing's syndrome include drug interactions and physiological/physiopathological changes in the hypothalamic-pituitary-adrenal axis.1–4 For instance, pseudo-Cushing's states mimic the clinical presentations of Cushing's syndrome and are associated with hypercortisolism, but disappear when the primary condition that led to the Cushing-like state resolves.18 Common causes of ‘pseudo-Cushing's syndrome’ include psychiatric disorders (depression, anxiety) and alcoholism. Certain medications may cause false-positive dexamethasone suppression tests by interfering with dexamethasone metabolism (diphenylhydantoin, carbamazepine, barbiturates) or increasing cortisol-binding globulin (estrogen, mitotane). Moreover, hypercortisolism may be present during certain situations including physical stress (hospitalisation, surgery and pain), malnutrition, anorexia nervosa and hypothalamic amenorrhoea.1–4
Biochemical evaluation
The diagnosis of hypercortisolism is rendered biochemically, using at least two different testing modalities from the following: 24-h urinary free cortisol (two measurements), late-night salivary cortisol (two measurements), 1-mg overnight dexamethasone suppression test and longer low dose dexamethasone suppression test (2 mg/d for 48 h).1 Interpretation of the test results is dependent on the assay method used and the expertise of an endocrine specialist is often necessary to clarify the caveats of each test.1–4 ,8 After biochemical confirmation of hypercortisolism, further laboratory testing is required to determine the aetiology of Cushing's syndrome (table 1). Corticotroph response is assessed by measuring plasma ACTH levels.2–4 ,8 A plasma ACTH concentration of less than 5 pg/mL (1.1 pmol/L) is in keeping with ACTH-independent Cushing's syndrome, while a concentration of more than 15 pg/mL (3.3 pmol/L) is suggestive of ACTH-dependent Cushing's syndrome.2–4 ,8 Borderline plasma ACTH values between 5 pg/mL and 15 pg/mL (1.1 pmol/L and 3.3 pmol/L) are equivocal, and a CRH stimulation test may be helpful in differentiating Cushing's disease from other aetiologies.2–4 ACTH and cortisol increases after intravenous administration of CRH is suggestive of Cushing's disease, whereas a blunted response is observed when the aetiology is adrenal.2–4 In ACTH-dependent hypercortisolism, CRH stimulation test is also used in conjunction with high-dose dexamethasone suppression test to differentiate between pituitary and ectopic ACTH overproduction; if CRH stimulation and high-dose dexamethasone suppression tests are positive, a petrosal sinus sampling test may not be warranted to confirm or exclude the diagnosis of pituitary ACTH oversecretion.2–4
Radiological considerations in Cushing's syndrome
After biochemical confirmation of hypercortisolism and ACTH status, radiological investigations provide a supplementary method for preoperative subtyping (table 1). In ACTH-dependent Cushing's syndrome, pituitary MRI is the modality of choice to detect a pituitary adenoma.2–4 However, given the fact that most cases of Cushing's disease are caused by a pituitary microadenoma (<1 cm) often not visualised on MRI, bilateral inferior petrosal sinus sampling has become the gold standard test to confirm or exclude pituitary ACTH oversecretion.2–4 ,15 Petrosal venous sampling is not required when dynamic testing results are concordant with a pituitary source and there is a clearly visualised pituitary lesion suggestive of an adenoma and measuring >6 mm.19 Generally, an inferior petrosal sinus-to-peripheral ACTH ratio at baseline of more than 2:1 and a gradient of more than 3:1 following CRH stimulation is indicative of Cushing's disease.2–4
Assessment of adrenocortical lesions
In ACTH-independent Cushing's syndrome, adrenal CT scan is the imaging modality of choice to localise and subtype adrenocortical lesions preoperatively (table 2).2–4 ,11 ,20–24 The radiological findings for adrenal hyperplasia are often non-specific, showing either enlarged (figures 2A and 3A) or normal-sized adrenal glands.11 ,21–24 Primary pigmented nodular adrenocortical disease occasionally presents as small nodules with hypodense pigment.24–26 Primary macronodular hyperplasia may present with massive bilateral adrenal enlargement with multiple non-pigmented nodules, ranging from 1 cm to 5 cm (figure 3A).27 Adrenocortical adenomas are typically solitary and appear homogeneous, small (<4 cm), with smooth borders and well-delineated margins (figure 4A).21–23 Adrenocortical carcinomas are usually larger in size (>4 cm) and present heterogeneous contents, irregular margins, and occasional areas of necrosis, haemorrhage and calcification (figure 5A; table 2).21–23 Given the fact that many adenomas have intracytoplasmic fat resulting in lower attenuation, measurement of the attenuation value is helpful in distinguishing adenomas from carcinoma; tumours with attenuation values below 10 Hounsfield units on non-contrast CT are indicative of adrenocortical adenomas.21–23 ,28 Additionally, on contrast-enhanced CT, an absolute contrast washout of more than 60% is suggestive of adenoma.21
Diffuse hyperplasia. CT shows bilaterally enlarged adrenal glands in a patient with Cushing's disease (A). The adrenal gland is also diffusely enlarged and displays a normal zonation. In contrast to ectopic ACTH-related diffuse hyperplasia, the zonation of the cortex is often conserved in pituitary ACTH-dependent hyperplasia (B).
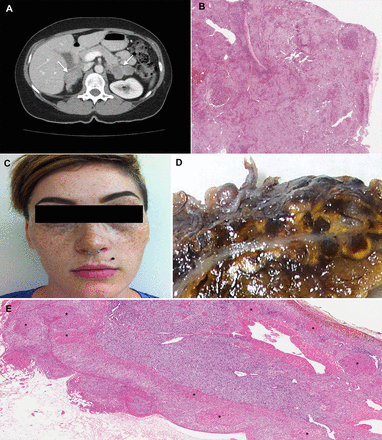
Nodular hyperplasia. Primary bilateral adrenocortical nodular hyperplasia is classified as either macronodular (nodules >1 cm) or micronodular (nodules <1 cm), although a degree of morphological overlap may occur. CT shows adrenal glands with primary bilateral macronodular adrenocortical hyperplasia (A). In primary bilateral macronodular adrenocortical hyperplasia, the external surface of the glands is often ‘bosselated’ in appearance. Cross-section of the gland often reveals uncapsulated yellow to golden-yellow nodules ranging from 1 cm to 5 cm with irregular light-brown foci. Microscopically, the cells are pale, lipid-rich and contain vacuolated cytoplasm, admixed with compact cells (B). The micronodular subtype presents a pigmented form ‘primary pigmented adrenocortical disease (PPNAD)’, and a scarcely/non-pigmented form ‘micronodular adrenocortical disease (MAD)’. Recognition of the pigmented variant (PPNAD) has clinical implications because it has been associated with 60% of patients with Carney complex. Photograph of a woman with Carney complex. Multiple ephelides are seen including some in the lips, which is a typical site of pigmentation in patients with this disorder (C). On cross-section, a large number of yellow to brown-black micronodules may be seen, ranging from 0.1 mm to 0.3 mm in size (D). Microscopically, the nodules (asterisks) are round-oval, encapsulated, and commonly found deep within the zona reticularis or near the corticomedullary junction (E).
Adrenal cortical adenoma. CT shows sagittal and coronal views of a cortisol-producing adrenal adenoma (A). In cortisol-producing neoplasms, the non-tumorous cortex almost inevitably shows adrenal cortical atrophy due to negative feedback suppression effect of the hypothalamic-pituitary axis (B).
Adrenal cortical carcinoma. CT shows sagittal and coronal views of a cortisol-producing adrenocortical carcinoma (A). Grossly, adrenocortical carcinomas may weigh more than 100 g, with measurements ranging from 3 cm to 40 cm (B). While some are encapsulated, many are adherent to or invade adjacent structures. On slicing, these tumours often reveal lobulation with fibrous bands and areas of necrosis and haemorrhage (B). Microscopically, adrenocortical carcinomas exhibit a more disorganised architecture than adenomas, with trabecular and diffuse patterns. Nuclear pleomorphism and increased mitoses (>5/50 high power fields), including atypical mitotic figures (C), are frequently seen with capsular and vascular invasion. Vascular invasion is diagnosed when intravascular tumour cells adherent to thrombus is identified (D). A recently described novel approach using a ‘reticulin algorithm’, which has been shown to have high interobserver reproducibility.60–62 In this method, malignancy is suggested by an altered reticulin framework (E) along with one of the following parameters: high mitotic rate (>5 mitoses per 50 high power fields), vascular invasion and necrosis. Moreover, insulin-like growth factor 2 (IGF2) and p53 overexpression (F), along with a high Ki-67 (>5%) have been described to be effective in differentiating carcinoma from adenoma.
Adrenal incidentalomas
With the advent of widespread imaging studies, primary hypercortisolism is increasingly diagnosed as part of the recommended workup for adrenal incidentalomas, which are detected for reasons unrelated to adrenal diseases.1 ,29–32 The reported incidence of adrenal incidentalomas is approaching the 8.7% incidence reported in autopsy series, with up to 10% of cases causing hypercortisolism.29 The most common presentation is subclinical Cushing's syndrome, although undiagnosed overt disease may also occur.29–32 While the management of subclinical Cushing's syndrome remains an area of controversy,29–32 the most recent guidelines suggest that adrenalectomy be offered to patients with worsening hypertension, abnormal glucose tolerance, dyslipidaemia or osteoporosis.29 Measurement of fractionated metanephrines and catecholamines is recommended in all cases of adrenal incidentalomas to rule out phaeochromocytoma.29 If hypertension is present, an aldosterone-to-renin ratio is also performed to exclude primary aldosteronism.28 ,29 ,33
Histopathological correlates of Cushing's syndrome
The normal adult adrenal gland weighs approximately 4 g at surgical excision, with an average cortical thickness of 2 mm.16 ,34–38 In Cushing's syndrome, histopathological correlates in the adrenal glands include hyperplasia, adenoma and carcinoma.11–13 ,34–39 While ACTH-dependent adrenal hyperplasia is the most common clinical manifestation of Cushing's syndrome, this entity rarely presents itself in surgical pathology because most cases are treated with transsphenoidal pituitary resection, ectopic tumour resection, radiation or medical therapy.2 ,3 ,14–16 Therefore, when confronted with adrenalectomy specimens in the setting of Cushing's syndrome, pathologists are most commonly exposed to cortisol-producing neoplasms and ACTH-independent macronodular and micronodular hyperplasia.11–13 ,16 ,34–38
Diffuse hyperplasia
Diffuse bilateral adrenocortical hyperplasia is seen in ACTH-dependent Cushing's syndrome (figure 2B), in response to excess pituitary or ectopic ACTH production, and very rarely ectopic CRH production.16 ,34 In late stages, a mixed diffuse and nodular adrenocortical hyperplasia has been described.34 In Cushing's disease, the spectrum of pituitary ACTH-producing adenomas includes densely or sparsely granulated corticotroph adenoma and Crooke's cell adenoma (table 1).40–42 Clinically, an inverse correlation between pituitary tumour size and symptomatology has been reported.41 ,42 Densely granulated corticotroph adenomas are usually described as microadenomas (<1 cm) causing the more florid Cushing presentation, whereas sparsely granulated corticotroph adenomas and Crooke's cell adenomas are often described as macroadenomas (>1 cm) presenting with subclinical Cushing features.41 ,42 The pathological assessment of Crooke's hyaline change in non-tumorous corticotrophs is clinically pertinent: if absent, the risk of recurrence after resection of a corticotroph adenoma is typically higher.41 ,42
Nodular hyperplasia
Primary bilateral adrenocortical nodular hyperplasia accounts for an estimated 10% of cases of adrenal Cushing's syndrome (figure 3).11–12 ,16 ,34 It is classified as either macronodular (nodules >1 cm) or micronodular (nodules <1 cm), although a degree of morphological overlap may occur.11–13 ,16 ,34
The macronodular subtype, previously known as ACTH-independent macronodular adrenocortical hyperplasia, has been recently redefined as primary bilateral macronodular adrenocortical hyperplasia (BMAH) due to the discovery of intra-adrenal production of ACTH in this lesion (tables 2 and 3).11–13 ,43–45 Clinically, BMAH has a slight male predominance, and tends to present in the fifth to sixth decade of life with subclinical Cushing's syndrome (table 2).11–13 However, a variant (c-BMAH; childhood BMAH) associated with McCune-Albright syndrome has been reported, arising in early childhood with more florid Cushing's syndrome presentation.6 ,11–13 Grossly, the adrenal glands appear enlarged and distorted, with a combined weight of 60–200 g.11 ,13 ,16 ,34 The external surface of the glands is often ‘bosselated’ in appearance. Cross-section of the gland reveals uncapsulated yellow to golden-yellow nodules ranging from 1 cm to 5 cm with irregular light-brown foci.11 ,13 ,16 ,34 Microscopically, the cells are pale, lipid-rich and contain vacuolated cytoplasm, admixed with rare compact cells containing strong eosinophilic cytoplasm (figure 3B). Minimal nuclear pleomorphism and rare mitotic figures are seen. The internodular cortex may be either hyperplastic or atrophic.11 ,13 Although this distinction is not well understood, the internodular cortical atrophy has been described in the McCune-Albright associated variant of BMAH (c-BMAH).11 ,13
Histopathological and molecular features of adrenal Cushing
The micronodular subtype presents a pigmented form ‘primary pigmented adrenocortical disease (PPNAD)’, and a scarcely/non-pigmented form ‘micronodular adrenocortical disease (MAD)’, although considerable clinical, morphological and genetic overlap is seen between the two groups (tables 2 and 3).11–13 ,34 ,46–49 Clinically, PPNAD and MAD have a slight female predominance; they also tend to arise at a younger age and with more florid Cushing features than BMAH. Biochemically, a paradoxical increase in the 6-day Liddle's dexamethasone suppression test has been described, which is thought to be correlated with glucocorticoid-receptor overexpression in the pigmented micronodules.50 Recognition of the pigmented variant (PPNAD) has clinical implications because it has been associated with 60% of patients with Carney complex (CNC, figure 3C), in which case echocardiography is warranted to rule out a potentially fatal cardiac myxoma.13 ,25 ,26 ,49 Grossly, adrenocortical micronodular hyperplasia presents with normal to slightly enlarged adrenal glands, with a combined weight of 4.3–17.0 g.11 ,12 ,16 ,34 On cross-section, a large number of yellow to brown-black micronodules may be seen, ranging from 0.1 mm to 0.3 mm in size (figure 3D). The pigmented appearance is a result of lipofuscin storage within the nodules (figure 3E). Microscopically, the nodules are round-oval, encapsulated, and commonly found deep within the zona reticularis or near the corticomedullary junction (figure 3D).34 ,47 The cells often show compact eosinophilic cytoplasm, although rare cells with lipid-rich and vacuolated cytoplasm may be seen.34 Mitotic figures are seldom present.34
The ancillary features of cortisol-producing adrenocortical nodular hyperplasia provide some clues as to why the macronodular subtype, despite massive adrenal enlargement, offer relatively inefficient cortisol overproduction in comparison with the normal-sized micronodular glands. Ultrastructural studies of macronodules often show poorly developed smooth endoplasmic reticulum in the large pale lipid-rich cortical cells, alongside weak reactivity for 3β-hydroxysteroid dehydrogenase and other enzymes involved in steroidogenesis.34 By contrast, cortical cells within smaller micronodules often show intense activity for steroidogenic enzymes. On ultrastructural studies, these cells show compact eosinophilic cytoplasm, with significantly more developed smooth endoplasmic reticulum.34
Adrenocortical neoplasms
Unilateral adrenocortical neoplasms account for 90% of cases of adrenal Cushing's syndrome.2–4 ,8–12 In these circumstances, the pathological assessment of the non-tumorous cortex is important to determine the functional status of the tumour. In cortisol-producing neoplasms, the non-tumorous cortex almost inevitably shows some degree of adrenal cortical atrophy due to negative feedback suppression effect of the hypothalamic-pituitary axis (figure 4B). In contrast, the non-tumorous cortex of non-functioning and aldosterone-producing adrenal cortical neoplasms is not atrophic. In aldosterone-producing neoplasms, the zona glomerulosa may even exhibit hyperplasia, the so-called ‘paradoxical hyperplasia’.16 ,28 ,34 ,51
In surgical series, adrenocortical adenoma is the most common adrenal presentation of Cushing's syndrome (figure 4), accounting for nearly 55% of cases (tables 2 and 3).2–4 ,11 ,12 ,16 Clinically, these tumours can arise at any age, with a slight female predominance.11 ,12 The presentation ranges from subclinical to overt Cushing.11 ,12 ,34 Smaller size adenomas, harbouring mutations in PRKACA and GNAS, have been reported with earlier onset and more overt hypercortisolism.52 Grossly, cortisol-producing adenomas are described as solitary, round-to-ovoid neoplasms, ranging from 1.5 cm to 6 cm in size, and weighing between 10 g and 40 g.16 ,34–37 On cross-section, the tumour is typically homogenous yellow or golden-yellow, with occasional foci of dark discolouration.16 ,34 Rare cases of pigmented ‘black’ adenomas have been reported, with a similar clinical course as their non-pigmented counterparts.16 ,34 Microscopically, the neoplastic cells tend to be pale-staining, lipid-rich, with uniform round-oval nuclei and clear cytoplasm.16 ,34–37 Common architectural patterns include nesting-alveolar, short cords, narrow interconnecting trabecular or a mixture of these patterns within the same tumour.34 Mitotic figures are rarely present.34–37 On electron microscopy, cortisol-producing adenomas contain mitochondria with tubular or vesicular cristae, which are quite distinct from the lamellar type or plate-like cristae seen in aldosterone-producing adenomas.28 ,34 ,51
In contrast to adenomas, adrenocortical carcinomas are quite rare and account for 35% of cases of adrenal Cushing's syndrome (tables 2 and 3).2–4 ,11 ,12 ,16 ,47 Clinically, they can present at any age depending on whether they arise sporadically or in the setting of familial syndromes.11 ,12 ,53–58 The more common sporadic cases tend to present in the fourth to fifth decade of life with a female predominance, aggressive onset of hypercortisolism, overt Cushing's syndrome features and occasionally concurrent virilisation symptoms.11 ,12 ,53–57 Grossly, adrenocortical carcinomas may weigh more than 100 g, with measurements ranging from 3 cm to 40 cm.16 ,28 ,34–38 ,59 While some are encapsulated, many are adherent to or invade adjacent structures. On slicing, these tumours often reveal lobulation with fibrous bands and areas of necrosis and haemorrhage (figure 5B). Microscopically, adrenocortical carcinomas exhibit a more disorganised architecture than adenomas, with trabecular and diffuse patterns.34–38 ,59 Nuclear pleomorphism and increased mitoses (>5/50 high power fields), including atypical mitotic figures (figure 5C), are frequently seen with capsular and vascular invasion in the vast majority of high-grade adrenocortical carcinomas.28 ,34–38
While the morphological features of ‘florid’ adrenal cortical carcinomas are quite different from typical benign adenomas, the distinction between a non-invasive low-grade carcinoma and an adenoma with atypical features can be challenging in some cases.28 ,58 ,60 Even the commonly used Weiss scoring system carries significant interobserver variability, along with diagnostic uncertainty when an adrenal cortical neoplasm receives an intermediate score of 2 or 3.28 ,58 ,60 Recently, Volante et al described a novel approach using a ‘reticulin algorithm’, which has been shown to have high interobserver reproducibility.60–62 In this method, malignancy is suggested by an altered reticulin framework (figure 5D) along with one of the following parameters: high mitotic rate (>5 mitoses per 50 high power fields), vascular invasion and necrosis.58 ,60–62 Moreover, IGF2 and p53 overexpression, along with a high Ki-67 (>5%) have been described to be effective in differentiating carcinoma from adenoma.28 ,34–38 ,58 Recent transcriptome analysis highlighted that adrenal cortical carcinomas can be distinguished clearly from adenomas slightly better than morphological assessment based on gene expression profile of an adrenal cortical neoplasm.54 ,58 ,63 Moreover, DNA microarray analysis of a large group of adrenocortical tumours divided the malignant tumours into two groups and provided prognostic information independent of tumour mitotic rate and stage.54 ,58 ,63 While the combined expression of BUB1B and PINK1 provided overall the best prediction of overall survival, the combined expression of DLG7 and PINK1 was found to be the best predictor of disease-free survival.63 Occasionally, when there is a need to differentiate between a primary or metastatic adrenocortical tumour, the adrenocortical origin can be demonstrated by positive staining of markers of adrenal cortical cytodifferentiation, which includes steroidogenic factor-1, Melan-A (clone A103), calretinin, synaptophysin and α-inhibin.28 ,34–38 ,58
Pathogenesis and molecular features of adrenal Cushing's syndrome
Over the last decade, significant advances made in molecular pathology have enhanced our understanding of Cushing's syndrome (figure 6). Recent application of whole-genome sequencing techniques have allowed the discovery of somatic and germline mutations implicated in the pathogenesis of primary cortisol-producing adrenocortical lesions.52 ,64–72 Most of these mutations (PRKACA, PRKAR1A, GNAS, PDE11A, PDE8B) cause aberrant activation of the cyclic AMP (cAMP)-signalling pathway, resulting in hormone overproduction and cellular proliferation (figure 6).11–13 ,52 ,64–81 Other mutations involve pathways associated with ‘neoplastic transformation’, favouring tumour growth over hormone overproduction.70 These include mutations in armadillo repeat containing 5 gene (ARMC5) a putative tumour suppressor gene, Wnt/β-catenin pathway (CTNNB1, ZNRF3), growth factor overexpression (IGF2), p53/retinoblastoma protein pathway (TP53, CDKN2A, RB1) and chromatin remodelling (MEN1, DAXX).11–13 ,39 ,56 ,58 ,82–91
Molecular biological features of adrenal Cushing. In normal physiology, cortisol secretion in adrenal zona fasciculata (ZF) cells is mediated by the cAMP/protein kinase A (PKA) signalling pathway. In the resting state, protein kinase A exists as an inactive tetramer, with the catalytic subunits (PKA-C) bound to regulatory subunits (PKA-R) (A). Under stress, the pituitary gland secretes corticotropin (ACTH), which binds to melanocortin receptor 2 (MC2R) on the ZF cells, causing activation of adenylyl cyclase through stimulatory G-protein α subunit (Gsα), generating cyclic AMP (cAMP) from ATP (B). The cAMP then binds PKA-R, causing release of PKA-C. This results in phosphorylation of downstream targets, including cAMP response element-binding protein (CREB), which induces cortisol biosynthesis and proliferation of ZF cells. After the stimulus finishes, cAMP is hydrolysed by phosphodiesterase (PDE) and the PKA subunit is reassembled again, returning to its inactive state. In adrenal Cushing, somatic and germline mutations may arise at various steps of the cAMP/PKA pathway, causing excess signalling and resultant cortisol overproduction and secretion. These include activating mutations in PKA-C (PRKACA), PKA-R (PRKA1A), Gsα (GNAS), MC2R (MCR2), inactivating mutations in PDEs (PDE11A and PDE8B) and ectopic/aberrant G-protein-coupled receptors (C).
Normal physiology of adrenal cortisol secretion
In normal physiology, cortisol secretion is under the control of the hypothalamic-pituitary-adrenal axis (figure 6A).12 ,52 ,64–70 Under stress, the hypothalamus releases CRH, which result in ACTH secretion from the anterior pituitary gland. ACTH binds to melanocortin-2 receptor, a G-protein-coupled receptor in the adrenal fasciculata cells, causing activation of adenylyl cyclase through Gs-α subunit, and generating cAMP (figure 6B).12 ,52 ,64–70 The cAMP then binds the regulatory subunit of protein kinase A, causing release of the PKA catalytic subunit (PKA-C). This results in phosphorylation of downstream targets, including cAMP response element-binding protein, which induces cortisol biosynthesis and proliferation of the adrenal cortex.52 After the stimulus finishes, cAMP is hydrolysed by phosphodiesterase and the PKA subunit is reassembled again, returning to its inactive state.12 ,52 ,64–70
Primary adrenocortical hyperplasia
The role of aberrant cAMP signalling (figure 6C) in autonomous adrenal cortisol production was initially described in primary adrenocortical nodular hyperplasia. Recent genetic analyses of these lesions have revealed distinct mutations implicated in the pathogenesis of macronodular and micronodular subtypes (table 3).4 ,11–13 ,27 ,49 ,64
Historically, the macronodular subtype, also known as primary BMAH, was thought to be mainly sporadic due to aberrancies in the cAMP signalling pathway.4 ,11–13 ,27 ,92 Cases with aberrant G protein-coupled membrane receptor (GPCR) response to a variety of hormones (vasopressin, serotonin, gastric inhibitory polypeptide, catecholamines, luteinising hormone/human chorionic gonadotropin, angiotensin) are well described in the literature.13 ,92 ,93 Rare activating mutations in melanocortin-2 receptor have also been reported.11–13 c-BMAH has been linked to a postzygotic activating mutation (somatic mosaicism) in the GNAS gene which encodes the Gα subunit in the cAMP pathway; c-BMAH occurs frequently in the setting of McCune-Albright syndrome but may also present in an isolated manner.11–13 ,27
Over the past year, our knowledge of BMAH has greatly evolved. The recent discovery of inactivating germline mutations (ARMC5) in over 50% of cases suggests that this disease is much more hereditary than previously thought.70 ,82–86 Mutations in the ARMC5 putative tumour suppressor gene has been shown to cause altered cell survival and decreased steroidogenesis, favouring the hypothesis of tumour growth over hormone production in the pathogenesis of BMAH.82–86 This is consistent with its common clinical presentation where relatively inefficient cortisol overproduction is seen despite massive adrenal enlargement.13 ,27 ,69 ,82–86 Very recently, a case of BMAH with ARMC5 mutation has also been described with concomitant intracranial meningiomas, raising the possibility of a new tumour syndrome.94 Other inherited cases have been reported in the setting multiple endocrine neoplasia type 1 syndrome (MEN1 gene), familial adenomatous polyposis (FAP) syndrome (APC gene), hereditary leiomyomatosis and renal cancer syndrome (FH gene), as well as familial alterations in cAMP signalling (familial GPCRs and PDE11A gene).11–13 ,27 Finally, recent studies have also shown that BMAH, traditionally thought to be ACTH-independent, is at least partially regulated by intra-adrenal production of ACTH.43–45 ,82 ,86
The micronodular subtype is mainly hereditary in nature, although rare sporadic cases have also been reported (table 3).11–13 ,46–49 The densely pigmented variant, known as PPNAD, typically occurs in the setting of Carney complex (c-PPNAD) and rarely in isolated cases (i-PPNAD). The pathogenesis of c-PPNAD has been attributed predominantly (up to 73%) to an inactivating germline mutation in PRKAR1A gene, which encodes the regulatory subunit of protein kinase A, and in rare circumstance to alterations of the CNC2 gene locus.11–13 ,46–49 ,64 The pathogenesis of i-PPNAD has been linked to inactivating mutations in PRKAR1A, as well as rare alterations in phosphodiesterase genes (PDE11A, PDE8B) and 2p16.11 ,12 ,49 Cases of scarcely or non-pigmented primary micronodular hyperplasia (MAD) have been associated with inactivating mutations in PDE genes (PDE11A, PDE8B), as well as alterations of 2p12-p16 and 5q.11 ,12 ,49 ,80 ,81
Cortisol-producing neoplasms
Contrary to the inherited nature of primary cortisol-producing adrenocortical hyperplasia, cortisol-producing adrenocortical neoplasms often arise sporadically (table 3). Rare cases of adrenocortical adenoma have been reported in the setting of multiple endocrine neoplasia type 1 syndrome (MEN1), FAP syndrome, McCune-Albright syndrome, CNC, Carney triad and hereditary leiomyomatosis and renal cancer syndrome.11 ,12 ,56 ,95 Moreover, cases of adrenocortical carcinoma have been reported in association with cancer susceptibility syndromes (Li-Fraumeni syndrome (TP53 gene), Beckwith-Wiedemann syndrome (alteration of 11p15.5; IGF2), MEN1, FAP, Lynch syndrome, neurofibromatosis type 1 syndrome) and CNC.11 ,12 ,47 ,53–58 A rare Brazil variant of adrenocortical carcinoma, involving a specific germline TP53 mutation (R337H), has been described, arising in early childhood.11 ,12 ,53–58
The pathogenesis of sporadic cortisol-producing adrenocortical adenomas has remained largely unknown until earlier this year when six independent studies consecutively reported somatic activating mutations in the catalytic subunit of PKA (PRKACA gene) in 35–69% of cases.52 ,64–72 Based on crystal structure studies of PKA, the mutation is proposed to disrupt the interface between catalytic (PKA-C) and regulatory subunits.64–72 This disruption results in unregulated PKA-C activity, which causes PKA hyperactivation and cortisol overproduction.52 ,64–72 Somatic activating mutations in the GNAS gene have also been reported in 5–17% of cases of adenomas.52 ,67 In contrast with PRKACA and GNAS mutations causing aberrant cAMP/PKA signalling, the other frequently reported mutation in cortisol-producing adenomas involves the CTNBB1 gene (16%), which results in excess Wnt/β-catenin signalling.39 ,52 Interestingly, aberrant activation of the Wnt/β-catenin pathway has also been reported in 70% of aldosterone-producing adrenocortical adenomas.28 ,96 In a recent study, mutations in PRKACA, GNAS and CTNBB1 appeared to be mutually exclusive, which might explain the distinct genotype-phenotype correlations seen in cortisol-producing adenomas.52 Adenomas harbouring mutations in the cAMP pathway (PRKACA and GNAS) are generally smaller in size and present with more overt hypercortisolism than those harbouring mutations in the Wnt/B-catenin pathway (CTNBB1).52 Rare cases of sporadic cortisol-producing adenomas have also been reported with alterations in PRKAR1A, PDE8B, and G protein-coupled receptors.11 ,12
In contrast with sporadic cortisol-producing adenomas, alterations in the cAMP pathway are scarcely reported in sporadic cortisol-producing adrenocortical carcinoma (table 3).11 ,12 The most frequently reported molecular alterations in adrenocortical carcinoma relate to ‘tumorigenesis’ pathways involving cellular proliferation, differentiation, survival and/or apoptosis.39 ,70 These include aberrant Wnt/β-catenin signalling (CTNNB1 and ZNRF3 gene mutations), insulin-like growth factor 2 (IGF2), p53/retinoblastoma protein signalling (TP53, CDKN2A and RB1 gene mutations), chromatin remodelling (MEN1 and DAXX) as well as alterations in MED12 and TERT. Rare sporadic carcinomas have also been reported with alterations in PRKAR1A.11 ,12 ,53–58 ,87 ,90 ,97
The topic of precursor lesions in adrenal neoplasms remains an area of controversy.46 ,98 In contrast with other solid tumours such as in colon cancer, the frequently seen hyperplasia-adenoma-carcinoma sequence has not been clarified in the adrenal cortex.58 ,98 ,99 Cases of adrenocortical adenomas and carcinomas arising in a background of hyperplasia have been frequently described in genetic syndromes (CNC, McCune-Albright syndrome, MEN1, FAP).46 ,47 Moreover, aberrancies in Wnt/β-catenin signalling are increasingly reported in the development of hyperplasia and subsequent adrenocortical adenomas and carcinomas.28 ,39 ,46 ,58 ,99 Recently, Ronchi et al99 provided the first genome-wide high-resolution study of chromosomal changes in a large series of adrenocortical tumours.58 They discovered that malignant tumours carry more genetic aberrations than benign ones, and over 70% of the most frequent genetic alterations (small isolated copy number gains) found in adenomas were also present in carcinoma, supporting the hypothesis of a common early molecular signature. These data suggest that there is likely a subset of adrenocortical carcinoma that arises in the background of adrenocortical adenoma and hyperplasia.
Treatment and prognosis
The management of endogenous Cushing's syndrome is mainly surgical and tailored to its primary aetiology.2–4 ,11–15 In ACTH-dependent Cushing's syndrome, first-line treatment modalities include transsphenoidal pituitary adenomectomy and ectopic corticotroph tumour resection.14 ,15 Second-line treatment is necessary when tumour resection is not possible or not curative. The pituitary gland may be addressed with radiation therapy and/or medical therapy using a dopamine agonist (cabergoline), or a somatostatin receptor agonist (pasireotide).100 ,101 Other options for controlling endogenous glucocorticoid excess include targeting of the adrenal gland via bilateral adrenalectomy or the use of steroidogenesis inhibitors (ketoconazole, metyrapone, mitotane, etomidate). Finally, the peripheral action of cortisol may be antagonised with mifepristone.102 Adrenalectomy is seldom used in ACTH-dependent Cushing because of the risk of Nelson's syndrome and lifelong adrenal replacement therapy.14 ,15 In contrast, the treatment of choice for ACTH-independent Cushing's syndrome is laparoscopic adrenalectomy.2–4 ,11 ,13 ,20 Bilateral adrenalectomy is recommended for adrenocortical micronodular and macronodular hyperplasia, while solitary cortisol-producing adrenocortical neoplasms are typically treated with unilateral adrenalectomy.3 ,11 ,25 Perioperative adrenal supplementation is often necessary to avoid Addisonian crisis, a potentially fatal complication.3 ,20 In BMAH, medical therapy may be attempted prior to surgery using steroidogenesis inhibitors or specific GPCR therapy (β-blocker, GnRH analogue, somatostatin agonist), and unilateral adrenalectomy has been proposed in milder cases.13 The use of laparoscopic techniques remains controversial in suspected cases of adrenocortical carcinoma, and many institutions recommend open surgery.11 ,54 ,55 ,20 There is ongoing debate regarding the indications for adjuvant mitotane therapy. Most investigators recommend it after incomplete resection of the adrenal carcinoma or in cases in which the carcinoma has a high proliferation (Ki67>10% or mitotic rate>20 per 50 high-power fields).54 ,55 ,103 Germline testing of p53 mutation is currently recommended in all patients diagnosed with adrenocortical carcinoma.47 ,54 ,104
Conclusions
Over the past year, important milestones in the understanding of endocrine disorders and tumorigenesis have been achieved through genomic sequencing of adrenal cortical tumours. Concurrent to the discovery of mutations (KCNJ5, ATP1A1, ATP2B3, CACNA1D) in the calcium/calmodulin kinase pathway causing primary aldosteronism,28 ,105 somatic and germline mutations (PRKACA, PRKAR1A, GNAS, PDE11A, PDE8B) discovered in the cAMP/PKApathway is now known to cause most cases of primary adrenal hypercortisolism. Despite emerging evidence of aberrant Wnt/β-catenin signalling in the development of adrenocortical hyperplasia and neoplasms, the frequently seen hyperplasia-adenoma-carcinoma progression sequence remains to be determined in the adrenal cortex. Further clarification of these molecular biological features is critical to better classify and understand the genotype-phenotype correlates in adrenal Cushing's syndrome. Pathological correlates include adrenocortical hyperplasia, adenoma and carcinoma. In conjunction with careful consideration of clinical, biochemical and radiological findings, a thorough pathological assessment is currently the gold standard for subtyping and management of Cushing's syndrome.
Take home messages
-
Accurate subtyping of Cushing's syndrome is essential for treatment decision-making and requires a complete integration of clinical, biochemical, radiological and pathological findings.
-
Pathological correlations of Cushing's syndrome in the adrenal glands include adrenal cortical hyperplasia (diffuse and nodular), adenoma and carcinoma.
-
Somatic and germline alterations in the cAMP/PKApathway is now known to cause most cases of primary adrenal hypercortisolism.
-
In cortisol-producing adrenal cortical neoplasms, the non-tumorous cortex almost inevitably shows adrenal cortical atrophy due to negative feedback suppression effect of the hypothalamic-pituitary axis.
-
The diagnosis of adrenal cortical carcinoma is suggested by an altered reticulin framework along with one of the following parameters: high mitotic rate (>5 mitoses per 50 high power fields), vascular invasion and necrosis.
-
IGF2 and p53 overexpression, along with a high Ki-67 (>5%) have also been described to be effective in differentiating adrenal cortical carcinoma from adenoma.
-
Transcriptome profile of an adrenal cortical neoplasm distinguishes carcinoma from adenoma and provides prognostic information in a definite carcinoma.
References
Footnotes
-
Contributors OM: substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. KD, KGH and OM: drafting the work or revising it critically for important intellectual content.
-
Competing interests None.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Errata