Article Text
Abstract
Primary aldosteronism (PA) is the most common form of secondary hypertension and is critical to identify because when caused by an aldosterone-producing adenoma (APA) or another unilateral form, it is potentially curable, and even when caused by bilateral disease, antihypertensives more specific to PA treatment can be employed (ie, aldosterone antagonists). Identification of unilateral forms is not generally accomplished with imaging because APAs may be small and elude detection, and coincidental identification of a non-functioning incidentaloma contralateral to an APA may lead to removal of an incorrect gland. For this reason, the method of choice for identifying unilateral forms of PA is selective adrenal venous sampling (AVS) followed by aldosterone and cortisol analysis on collected samples. This procedure is technically difficult from a radiological standpoint and, from the laboratory perspective, is fraught with opportunities for preanalytical, analytical and postanalytical error. We review the process of AVS collection, analysis and reporting. Suggestions are made for patient preparation, specimen labelling practices and nomenclature, analytical dilution protocols, which numerical results to report, and the necessary subsequent calculations. We also identify and explain frequent sources of confusion in the aldosterone and cortisol results and provide an example of tabular reporting to facilitate interpretation and communication between laboratorian, radiologist and clinician.
- Aldosterone
- Adrenal Gland
- Renal
- Veins
Statistics from Altmetric.com
Introduction
Primary aldosteronism (PA) is the most common form of secondary hypertension accounting for approximately 6%–11% of unselected hypertension cases.1 2 The identification of patients with PA is critical because the condition is treatable and often curable, allowing patients to avoid the well-documented sequelae of long-standing hypertension. Though extensive clinical practice guidance for the testing, diagnosis and management of PA has existed for some time,3 4 there is comparatively little published, beyond analytical methodologies and assay validation, relating to the laboratory aspects of PA investigation.5 6
Biochemical case-finding for PA is accomplished with the simultaneous determination of plasma aldosterone concentration and either plasma renin activity or plasma renin concentration followed by the calculation of the aldosterone-to-renin ratio (ARR). The ARR is compared with a screening threshold specific to both the aldosterone and renin methods.4 5
Diagnostic confirmation of PA, though not always considered necessary,7 is established by one of a number of dynamic function tests including intravenous saline suppression, fludrocortisone suppression, oral salt loading and captopril suppression.4 8 9 After confirmation of the diagnosis, identification of unilateral forms, the vast majority of which are caused by aldosterone-producing adenoma (APA), is necessary, as these require surgical intervention if a cure is sought.
Most APAs are small and many small enough to evade radiological detection by CT or MRI, meaning patients with APA may have apparently normal adrenal glands on imaging.10 11 Additionally, unilateral forms of PA caused by microscopic aldosterone-producing cell clusters are also amenable to curative surgery and should not be excluded from diagnostic consideration.12
At the same time, because non-functioning adrenal adenomata are common,13 particularly in the elderly, the identification of an adrenal adenoma using imaging is not proof-positive that the affected gland is responsible for PA. It is known that the accuracy of CT and MRI for the identification of APA is poor,14 and results may even be misleading.15 For example, a patient could have bilateral idiopathic adrenal hyperplasia (IAH) with a coincidental unilateral adrenal adenoma or could have a non-functioning adrenal adenoma and an unvisualised contralateral APA. For these reasons, it is recommended that patients with PA undergo bilateral adrenal venous sampling (AVS) with analysis of adrenal vein (AV) and inferior vena cava (IVC) samples for both cortisol and aldosterone.4 Note, however, that clinicians may elect to refer younger patients (<35 years) with clinical features more suggestive of APA (overt hypokalaemia and low-density unilateral adrenal adenoma on CT) directly to surgery without AVS.
The laboratory preanalytical, analytical and postanalytical process for AVS is fraught with opportunities for error that could negatively affect results. Since AVS aldosterone and cortisol results are used to direct surgery, it is critical that the laboratory manages AVS collections correctly. In this best practice article, we will address the essential role laboratorians play in proper patient preparation, specimen labelling and handling, analysis, resulting and interpretation.
AVS process overview
AVS is performed by interventional radiology under fluoroscopic guidance and is technically challenging by virtue of the anatomy of the right adrenal vein (RAV).16 While the left adrenal vein (LAV) inserts into the left renal vein, which serves as an anatomical landmark making LAV samples comparatively easy to collect, in contrast, the RAV inserts directly into the IVC in the vicinity of other veins of similar calibre such as the right hepatic accessory and renal capsular veins. It is shorter in length and once cannulated, the catheter may therefore fall out under the motion of breathing. The RAV also tends to collapse under aspiration. This means that it is more common to cannulate and sample effluent from an incorrect vein on the right and even when cannulated, the RAV is more challenging to sample.
Some sites use infusion or bolus of (1-24)adrenocorticotropic hormone (ACTH) to increase aldosterone and cortisol production during AVS to improve biochemical confirmation of successful cannulation and to overwhelm any stress-related pulsatility in the adrenal production of aldosterone and cortisol.4 The advantages and disadvantages of ACTH are discussed below. Irrespective of the use of ACTH, samples for aldosterone and cortisol analysis are collected from the RAV, LAV and peripheral blood (IVC, femoral or antecubital fossa). For simplicity, peripheral blood collections will be referred to herein as collected from the ‘IVC’.
It is recommended that AV samples be collected from the RAV and LAV simultaneously when ACTH is not employed,17 because stress-induced fluctuations in adrenal steroid production can be seen with sequential collections.18 In practicality, because right-sided collections are challenging, there may still be a brief gap between RAV and LAV collections even when bilateral cannulation is employed.
Biochemistries, rather than radiological images, are used to determine the technical success of AVS. The ratio of RAV and LAV cortisol to IVC cortisol is used to assess whether cannulation (‘selectivity’) has been achieved. This ratio is called the selectivity index (SI; equation 1):
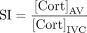 (1)
(1)
The SI threshold deemed demonstrative of successful cannulation ranges from as low as 1.1 to as high as 3,19 and higher if ACTH is used. While this continues to be debated in the literature, based on our outcome-adjudicated analysis of our local experience using different SI thresholds,20 our institutions employ SI≥2 to define selectivity in the absence of ACTH and SI≥3 for ACTH-simulated collections.21 22 Samples that do not meet the criteria for adequate AV placement should not be subjected to further clinical interpretation. If the procedure is deemed successful (‘bilaterally selective’) based on respective SI of the right and left, then the aldosterone:cortisol (A/C) ratios from the LAV and RAV are compared to determine whether aldosterone production is unilateral (making surgery a possibility) or bilateral (limiting treatment to medications). This ratio-of-ratios (the larger, ‘dominant’, A/C ratio divided by the smaller, ‘non-dominant’, A/C ratio) is called the lateralisation index (LI; equation 2) and values greater than 3–5 have been used to define lateralised aldosterone production.
 (2)
(2)
From the earliest days of AVS, unilateral versus bilateral PA has been defined primarily by this LI concept even if it is largely based on theoretical considerations (as there is no reference-interval data derived from patients without PA). In recent years, investigators have shown increased interest in the use of a ‘secondary’ interpretative criterion, the ‘contralateral suppression index’ or CSI (equation 3). The CSI derived from the observation that, in cases of APA, the non-dominant or uninvolved adrenal was often noted to be ‘suppressed’ to the point where aldosterone output was even less than the aldosterone measured in an IVC sample.
 (3)
(3)
A CSI is deemed to be positive (suppression present) when it is <1.0–1.4 and, at this level, appears to have a positive predictive value for APA that is very similar to the standard LI.23 24 There is no agreement on whether the LI or the CSI is the preferred AVS interpretation; at our institutions, we have traditionally used the LI, and the CSI is viewed as a secondary criteria that strengthens the overall APA diagnosis when present.
The challenge of RAV collections leads to poor technical success rates for AVS at some sites, as low as 10%.21 25 Not surprisingly, experience is important, and it is therefore recommended that centres performing AVS limit the number of operators so that they can achieve a critical number of cases yearly. The keys to successful AVS from a radiological perspective are effectively reviewed elsewhere16 26 and will not be covered here.
While other biochemical markers are currently under exploration for improved PA subtyping27 or as superior markers of selectivity,28 29 and while ACTH is not the only tool for adrenal stimulation,30 31 we will focus our attention to established approaches and the standard laboratory analysis of AVS collections.
The preanalytical phase
Patient preparation
It is not uncommon for our laboratorians to be consulted regarding the effects of medication on the aldosterone and cortisol analyses of the AVS procedure in the same manner as questions arise about medications and the ARR.32–34 While the effect of antihypertensives and other medications on AVS has not been systemically studied, a general principle is applied, namely, that those medications increasing renin (diuretics, ACE inhibitors, angiotensin receptor blockers (ARBs) and aldosterone antagonists) may result in stimulation of an unaffected gland contralateral to an APA and thereby result in factitious loss of lateralisation and misclassification of a patient with APA as IAH.4 17 It is also important that the patient be rendered eukalaemic prior to AVS as hypokalaemia decreases aldosterone synthesis.35 Many clinicians are not aware of the preanalytical effects of medications, and it is recommended that the AVS coordinating centre provide clinicians a checklist to encourage appropriate patient preparation.
Standardisation of order of collection
Ideally, AVS specimens should always be collected, accessioned and analysed in the same order. We recommend that this order be RAV, LAV, then IVC. Even when RAV and LAV are simultaneously cannulated, if they cannot be simultaneously aspirated because the RAV demands the radiologist’s full attention, the RAV should be sampled first before turning to the LAV. The motivation for doing RAV before LAV is that RAV is always more technically challenging and therefore if RAV is performed first, the respective collections will be close in their collection time and be more useful for comparative calculation purposes.26 However, if the LAV is collected first ‘because it’s easier’, the RAV may represent a challenge and its collection may be delayed, in which time the patient may become stressed increasing cortisol and aldosterone production leading to potential misclassification.
Standardised communication
One approach we have found effective is that the interventionalist verbally confirm the source, timing and placement of each individually collected AVS sample with an attending technologist, in duplicate, prior to moving to another collection. Institution of this meticulous procedure has eliminated mislabelling during collection.
Unique timestamps
Every specimen collected from a different anatomical site should be assigned a unique time of collection on its label. This affords a redundancy in the labelling allowing confirmation of specimen identification in the case of downstream mislabelling. This is important because specimens may require relabelling (or rather, overlabelling) at multiple stages in the lab process: in accessioning and if aldosterone is a send-out test, again in accessioning of the referral lab. If an absurd result is obtained by the reporting lab (eg, an IVC aldosterone and cortisol of 55 000 pmol/L and 1340 nmol/L respectively), the presence of a unique timestamp helps identify the root cause of the error and to determine whether it is safe to relabel and report the results rather than repeat the expensive and time-consuming AVS procedure.
Standardised nomenclature and prelabelling
Sites performing AVS should establish standardised labelling nomenclature for AVS and consider prelabelled tubes in the anticipated order provided this suits the radiologists’ preferences. For example, tubes could be prelabelled as: right adrenal, left adrenal and IVC, or if collections are performed pre-ACTH and post-ACTH, the labelling could be: right adrenal pre-ACTH, left adrenal pre-ACTH and IVC pre-ACTH, right adrenal post-ACTH, left adrenal post-ACTH and IVC post-ACTH. This means that the expected number of specimens is the same each time, the labelling becomes predictable to accessioning staff and technologist staff, and the chance of error becomes naturally lessened.
Laboratory information system (LIS) batteries
It is helpful to create a battery in the LIS specific for AVS reporting. This allows the systematic recording of all the information necessary for reporting and interpretation of the results. It also allows AVS specimens to be reported without the reference intervals that apply routine peripheral collections, which would be inappropriate. At our institution, the LIS battery contains the following data:
the sample series number from the AVS collections: from 1 to 6
the anatomical location of the collection (as per the specimen labelling)
the aldosterone result
the cortisol result
the AVS interpretation, which can be suppressed on specimens for which it is unnecessary.
When helping can hurt
It has been our experience that endocrinologists and/or radiologists may be under the impression that duplicate back-to-back collections from the AVs are de facto helpful. We have found that this is not always the case. Certainly, if there is a compelling reason to provide a second collection of the same sample (eg, if the cannula slipped during a first collection, or if the first sample has inadequate volume), then it is necessary to provide a second. However, if an identical second collection is provided for its own sake, then these extra collections create more complexity in specimen handling, labelling, analysis and reporting and increase the risk of error—particularly if routinely only one collection is expected. To be clear, however, if there is more than one anatomical RAV candidate, independent collections of each are mandatory in which case those should be clearly identified by radiology as collected from distinct anatomical locations.
We have also noticed that some radiologists may collect renal vein specimens or duplicate IVC collections from above and below the renal veins believing that these may show aldosterone gradients should the AV collections be non-selective. We have found collections like these to be diagnostically unhelpful for determining aldosterone lateralisation and have observed that they lead to confusion in accessioning, the bench and during interpretation. For example, accessioning staff may misread ‘renal’ as ‘adrenal’ or vice versa and mislabel.
We therefore advise that when it is achievable, a single good quality sample from the RAV, LAV and IVC is preferable to multiple identical collections and that collections from non-standard anatomical locations are to be discouraged. This means that three samples are generally expected, or if collections are performed pre-ACTH and post-ACTH, six.
ACTH or not?
ACTH administration prior to sample collection comes with costs and benefits. The benefit is that ACTH increases the concentration of both aldosterone and cortisol from the AV collections by one to two orders of magnitude and accordingly makes biochemical confirmation of AV selectivity more probable.22 26 36 37 Additionally, ACTH, by bolus or infusion, overwhelms any endogenous ACTH stimulation arising from patient discomfort, which might cause misleading results should one gland be under differential endogenous ACTH stimulation than the other.
The significant cost is that in lateralised PA cases (mostly APA), ACTH stimulates the otherwise suppressed gland leading to apparent decreases aldosterone lateralisation.21 38–40 The use of ACTH may therefore result in cases of APA being incorrectly identified as IAH. It is important to note that while the median LI decreases with the use of ACTH, there are certainly cases where it is higher post-ACTH than pre-ACTH. In any case, the question of whether to use ACTH is not completely resolved, some studies finding it of no advantage,41 while others advocating its use in centres with less-experienced radiologists.42 Because there are both costs and benefits, we collect samples without ACTH and then perform repeat AV collections 10 min post 250 μg/L IV bolus as do others.43 44 This allows the benefits while mitigating the costs but does create the possibility that the pre-ACTH and post-ACTH collections could have different interpretations, the latter being more likely appear bilateral.21 40 45 However, it has been our experience that ACTH rescues some procedures from being otherwise uninterpretable, for example, when only the post-ACTH collections are selective but show lateralisation.21
Intraprocedural cortisol
In recent years, some labs have advocated in the use of intraprocedural cortisol analysis to prove bilateral AV cannulation before completion of the AVS procedure.46–50 This is offered in a manner similar to intraoperative PTH analysis during parathyroidectomy. Those advocating this approach have sometimes employed a technologist and suitably small immunoassay analyser in the fluoroscopy suite (eg, Tosoh AIA-360),46 or have transported the specimen to the core laboratory for ‘stat’ analysis.47 48 The benefit of this service is it increases procedural success rates, provides immediate feedback to the radiologist and thereby helps train less-experienced operators. The challenges are financial and logistical: the availability of a suitable analyser (which, as reports indicate, are not usually point-of-care style analysers), coordination with technologists, use of their undivided time and the potential delay in completing the AVS case waiting for results since analyser incubations times are often 20 min. If analysis is not performed at point of care, there are also delays in transport, accessioning, centrifugation and reporting time. While we do not personally perform intraprocedural cortisol analysis, we acknowledge its value and it is worth consideration.
Predilution
Samples from successfully cannulated AVs, in terms of order-of-magnitude, usually show cortisol results in the 102–103 nmol/L range, while post-ACTH are usually in the 103–104 nmol/L range. Corresponding aldosterone concentrations are obviously contingent on whether the gland is affected by IAH, APA or is suppressed but range from 102–104 pmol/L in the absence of ACTH and 103–105 pmol/L (but occasionally 106 pmol/L) after ACTH. This means that dilution is almost always necessary for AV collections. The choice of diluent is not of major concern provided it is compatible with the assay since the results are ultimately interpreted on a relative, not an absolute, basis. Analysing all AV samples neat, at 10× and 50× dilutions has been recommended.26 This approach or similar is sound.
The effect of the dilution matrix should not be entirely ignored, however, as analytes may over-recover or under-recover at dilution. It is therefore prudent to report results from the LAV and RAV at the same dilution where possible and preferably at the dilution placing the raw concentration nearest to the middle of the analytical measurement range. This is ultimately a judgement call on the part of the laboratorian. The use of liquid chromatography and tandem mass spectrometry (LC-MS/MS) with its linear calibration curves for small molecules and its generally wider analytical ranges51 52 may allow for fewer dilutions.
Analytical phase
Specimens should be analysed in the same order as they are collected. This keeps the process simple, predictable and naturally less error-prone. Samples could be prepared for analysis in the order suggested in the online supplementary file 1 table.
Supplementary file 1
At one of our labs (St. Paul’s Hospital), we use LC-MS/MS for the simultaneous determination of aldosterone and cortisol.53 54 We have therefore dispensed with multiple dilutions and only perform analyses neat and 50× dilution.
Post-analytical
AV results can sometimes be confusing, and it is helpful to review some of the biochemical patterns that can be seen.
Low IVC results
Unstimulated aldosterone results from the IVC may be surprisingly low, even lower than the lowest recommended saline suppression test threshold of 138 pmol/L (5 ng/dL).4 These low results may cause confusion, as they seem inconsistent with a diagnosis of PA. However, the phenomenon is fairly common. For example, in a series of 443 non-adrenal (IVC, femoral and renal) AVS specimens collected from 355 unique patients and analysed at one of our institutions, 31 (6.9%) were below 138 pmol/L, 13 of which demonstrated aldosterone lateralisation (LI>4). An example of such a case is provided in table 1.
Suggested presentation of AVS data for report to clinician and radiologist. Data are from a 40-year-old female who presented with hypertension and low to low-normal potassium (K=3.0–3.9 mmol/L). Her screening plasma aldosterone was 882 pmol/L with a renin concentration of 2.6 ng/L for an ARR of 339 (N < 50). Saline suppression was performed as per standard approach.4 Post-saline aldosterone was 135 pmol/L (N < 138 pmol/L, 5 ng/dL). Given the borderline response to saline, the strongly positive screening data and the ongoing hypokalaemia, AVS was pursued, which showed clear left lateralisation. CT scan showed normal adrenals but possible thickening of the medial limb of the left adrenal without nodularity. Left adrenalectomy showed a 0.7 cm well-circumscribed lipid rich adenoma. The data illustrate a number of instructional points described above.
Low IVC results may be attributable to a few factors. First, though not recommended practice, the patients may have had aldosterone-lowering antihypertensives (particularly, ACE inhibitors, ARBs and beta blockers) reinitiated before the procedure. Additionally, the patients may have been supine for several hours before and during the procedure and, to a lesser extent, have had an intravenous running and have been given sedation with narcotic or benzodiazepine. In any case, laboratorians should expect to see this phenomenon from time to time.
LAV samples showing non-selectivity
While the LAV is easier to cannulate than the RAV, the LAV results may show lower cortisol levels than the RAV, indicating unexpectedly poorer sampling of the LAV effluent. The reason for this phenomenon is that the LAV forms a confluence with the inferior phrenic vein16 meaning that if LAV effluent is sampled from the confluence, it will be diluted with blood whose cortisol and aldosterone concentrations are similar to the peripheral blood.
Aldosterone depletion from putative RAV samples
On occasion AV samples from a RAV candidate are depleted in aldosterone relative to the IVC. They may even have undetectable aldosterone results. However, while their cortisol results will be lower than the IVC, cortisol will be relatively preserved. This phenomenon occurs when the accessory hepatic vein is accidentally cannulated instead of the RAV. The accessory hepatic is present in about 51% of the population55–57 and shares a common trunk with the RAV in about 12% of patients.58 For this reason, accidental sampling of hepatic effluent is seen and biochemically characterised by relative aldosterone depletion59 caused by the hepatic formation of tetrahydroaldosterone, its glucuronides and the 18-glucuronide of aldosterone.60 61
Closing the feedback loop
Finally, we must emphasise the value of providing the data to clinicians in an easily interpretable manner. LISs are generally designed to deliver discrete biochemical results. However, in the case of AVS, the results must be interpreted in light of one another and are much better represented in a tabular format to facilitate the SI and LI calculations. We therefore suggest that AVS results are prepared in a tabular format (see table 1) with SI and LI calculations provided and reported as a letter to the clinician from the laboratorian, in our case with interpretive advice using a standard approach. The radiologist who performed the procedure also receives a copy, which provides them immediate feedback and an opportunity to refine their approach should the procedure be unsuccessful or marginal. This facilitates the exchange of clinical information from clinician and radiologist to laboratorian allowing each to share experience and knowledge. Ultimately, the clinician must correlate the AVS findings to the clinical picture: the presence and location of a known adrenal mass, the age of the patient, the presence or absence of hypokalaemia and other factors may serve to increase or decrease the clinician’s confidence in the categorisation afforded by the AVS results.62 It is our experience that this communication between diagnosticians and clinicians has led to marked improvements in AVS success rates and ultimately to numerous cures.
Conclusion
AVS is an expensive procedure with a small but measurable complication rate of 0.61%63 and sometimes has a protracted clinical workup preceding it. For these reasons, the laboratory needs to do all it can to facilitate error-free collection, labelling, transport, accessioning, analytical and reporting processes so that repeat AVS is infrequently necessary. Laboratorians should work with clinicians and radiologists to establish whether ACTH stimulation should be employed and to determine a standardised collection order and specimen nomenclature allowing for predictable workflows to yield the best possible AVS results. Analytical phases should also be standardised, including AV sample predilution protocols, and we recommend that laboratorians consider providing customised tabular reports to both the clinician and the interventional radiologist. These steps allow for the identification of those patients who are most likely to benefit from unilateral adrenalectomy and cure.
Acknowledgments
The authors would like to acknowledge the clinical laboratory and radiology staff at their respective institutions, which make their PA programmes possible.
References
Footnotes
Handling editor Tahir S Pillay
Twitter Follow Daniel T Holmes @DrDanHolmes
Contributors DTH and GK both contributed to the conception, writing and editing of this manuscript.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.



