Summary
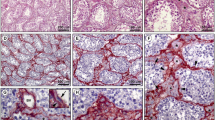
The lamina propria of human seminiferous tubules is composed of 5 to 7 cellular layers separated by laminae of extracellular connective-tissue components. By means of immunocytochemical methods the different nature of the cellular layers could be defined for the first time. Based on the light-microscopic demonstration of both desmin-like and vimentin-like immunoreactivity in the inner 3 to 4 layers of the lamina propria, these cells can be identified as myofibroblasts. The outermost one or two cellular layers, on the contrary, only show a vimentin-like immunoreactivity indicating the pure fibroblastic nature of these cells. Therefore, the outermost cellular layers are suggested to be derivatives of the interstitium. In cases of disturbed spermatogenesis, the lamina propria is frequently considerably thickened by an increase in the extracellular matrix components between the cellular layers. Whereas the ultrastructural localization of laminin-, collagen type-IV- and fibronectin-like immunoreactivity remains unaffected in the thickened lamina propria, the desmin-like immunoreactive cells of the inner layers strongly decrease in number and staining intensity. Most probably, the myofibro-blasts lose their myoid characteristics to participate in the secretion of increased amounts of extracellular matrix components, which in turn presumably block the mediation of the lamina propria between the interstitium and the germinal epithelium. It is still unclear whether the thickened lamina propria provokes the disturbance of spermatogenesis or vice versa.
Similar content being viewed by others
References
Baillie AH (1964) Ultrastructural differentiation of the basement membrane of the mouse seminiferous tubule. Q J Microsc Sci 105:203–207
Böck P, Breitenecker G, Lunglmayr G (1972) Kontraktile Fibroblasten (Myofibroblasten) in der Lamina propria der Hodenkanälchen vom Menschen. Z Zellforsch 133:519–527
Bustos-Obregón E (1974) Description of the boundary tissue of human seminiferous tubules under normal and pathological conditions. Verh Anat Ges 68:197–201
Bustos-Obregón E (1976) Ultrastructure and function of the lamina propria of mammalian seminiferous tubules. Andrologia 8:179–185
Bustos-Obregón E, Courot M (1974) Ultrastructure of the lamina propria in the ovine seminiferous tubule. Cell Tissue Res 150:481–492
Bustos-Obregón E, Holstein AF (1973) On structural patterns of the lamina propria of human seminiferous tubules. Z Zellforsch 141:413–425
Christl HW (1990) The lamina propria of vertebrate seminiferous tubules: A comparative light and electron microscopic investigation. Andrologia 22:85–94
Christl HW, Holstein AF, Becker H (1988) Das Verhalten der Lamina propria bei der Degeneration von Hodenkanälchen. In: Holstein AF, Leidenberger F, Hölzer KH, Bettendorf G (eds) Carl Schirren Symposium: Advances in Andrology. Diesbach, Berlin, pp 90–100
Clermont Y (1958) Contractile elements in the limiting membrane of seminiferous tubules of the rat. Exp Cell Res 15:438–440
DeKretser DM, Kerr JB, Paulsen CA (1975) The peritubular tissue in the normal and pathological human testis. An ultrastructural study. Biol Reprod 12:317–324
Fawcett DW (1973) Observations on the organization of the interstitial tissue of the testis and on the occluding cell junctions in the seminiferous epithelium. In: Raspé G, Bernhard S (eds) Advances in the biosciences, vol 10: Contraception: The masculine gender. Pergamon Vieweg, Oxford Edinburgh New York Toronto Sydney Braunschweig, pp 83–99
Hadley MA, Dym M (1987) Immunocytochemistry of extracellular matrix in the lamina propria of the rat testis: Electron microscopic localization. Biol Reprod 37:1283–1289
Hadley MA, Jarow JP, Marshall FF, Dym M (1985) Immunocytochemistry of the extracellular matrix in the human testis. J Androl 6:41a
Hermo L, Lalli M, Clermont Y (1977) Arrangement of connective tissue components in the walls of seminiferous tubules of man and monkey. Am J Anat 148:433–446
Holstein AF, Roosen-Runge EC (1981) Atlas of human spermatogenesis. Grosse, Berlin
Holstein AF, Roosen-Runge EC, Schirren C (1988) Illustrated pathology of human spermatogenesis. Grosse, Berlin
Hsu SM, Raine L, Fanger H (1981) The use of avidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase techniques. Am J Clin Pathol 75:816–821
Ito S, Winchester RJ (1963) The fine structure of the gastric mucosa in the bat. J Cell Biol 16:541–549
Mori H, Shiraishi T, Matsumoto K (1978) Ectopic Leydig cells in seminiferous tubules of an infertile human male with a chromosomal aberration. Andrologia 10:434–443
Roosen-Runge EC (1951) Motions of the seminiferous tubules of rat and dog. Anat Rec 109:413
Schulze C, Holstein AF (1978) Leydig cells within the lamina propria of seminiferous tubules in four patients with azoospermia. Andrologia 10:444–452
Schulze W (1979) “Divertikel” der Samenkanälchen des Menschen. Verh Anat Ges 73:693–694
Seidl K, Holstein AF (1990a) Evidence for the presence of nerve growth factor (NGF) and NGF receptor in human testis. Cell Tissue Res 261:549–554
Seidl K, Holstein AF (1990b) Organ culture of human seminiferous tubules: A useful tool to study the role of nerve growth factor in the testis. Cell Tissue Res 261:539–547
Skinner MK, Fritz IB (1986) Identification of a non-mitogenic paracrine factor involved in mesenchymal-epithelial cell interactions between testicular peritubular cells and Sertoli cells. Mol Cell Endocrinol 44:85–97
Skinner MK, Tung PS, Fritz IB (1985) Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol 100:1941–1947
Söderström KO (1986) Tubular hyalinization in human testis. Andrologia 18:97–103
Sternberger LA, Hardy PH Jr, Cuculis JJ, Meyer HG (1970) The unlabelled antibody method of immunohistochemistry. Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antiperoxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Tung PS, Fritz IB (1980) Interactions of Sertoli cells with myoid cells in vitro. Biol Reprod 23:207–217
Tung PS, Fritz IB (1986) Cell-substratum and cell-cell interactions promote testicular peritubular myoid cell histotypic expression in vitro. Dev Biol 115:155–170
Virtanen I, Kallajoki M, Närvänen O, Paranko J, Thornell LE, Miettinen M, Lehto VP (1986) Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec 215:10–20
Wrobel KH, Mademann R, Sinowatz F (1979) The lamina propria of the bovine seminiferous tubule. Cell Tissue Res 202:357–377
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davidoff, M.S., Breucker, H., Holstein, A.F. et al. Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res 262, 253–261 (1990). https://doi.org/10.1007/BF00309880
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00309880