Abstract
Background: Human breast cancers progressively grow despite the presence of extensive lymphocytic infiltration and specific antitumor immune recognition, thereby calling into question the competency of breast tumor-infiltrating lymphocytes (TIL). The function of breast TILs in vivo and their possible role in the suppression of an antitumor immune response are largely unknown.
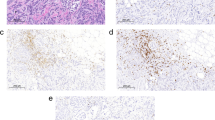
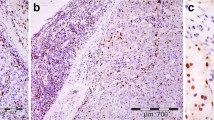
Methods: The cytokines produced in situ by lymphocytes in 89 breast carcinomas and 14 benign breast lesions were assessed using immunohistochemistry.
Results: The majority of tumor and benign breast samples contained T-cell infiltrates, which were disclosed using an anti-CD3 antibody stain. The percentage of tumor samples in which ⩾3% of the lymphocytes were producing cytokines was as follows: interleukin (IL)-2 45%, IL-4 36%, tumor necrosis factor-alpha (TNF-α) 28%, transforming growth factor-beta 1 (TGF-β1) 20%, IL-10 11%, interferon-gamma (IFN-γ) 4%, and granulocytemacrophage colony-stimulating factor (GM-CSF) 3%. Production of IL-2, IL-4, and TGF-β1 by TILs in breast cancers exceeded that detected in benign breast lesions (p<0.005). Significantly more tumor samples contained lymphocytes producing IL-2, IL-4, TGF-β1, and TNF-α than IFN-γ and GM-CSF (p<0.002 for each comparison). One or more of the potentially immunoinhibitory cytokines—IL-4, IL-10, or TGF-β1—were produced by lymphocytes in 44% of the specimens. No significant associations were seen between lymphocyte production of a particular cytokine and disease-free survival (median follow-up 43 months).
Conclusions: Immunohistochemical techniques can be used to detect cytokine secretion by TILs in preserved tissue. The relative lack of secretion of IFN-γ and GM-CSF, rather than a deficiency of IL-2, may explain why the antitumor immune response to breast cancer is impaired.
Similar content being viewed by others
References
Eremin O, Coombs RA, Prospero TA, Plumb D. T-lymphocyte subpopulations infiltrating human mammary carcinomas.J Natl Cancer Inst 1982;69:1–8.
Shimokawara I, Imamura M, Yamanaka N, Ishii Y, Kikuchi K. Identification of lymphocyte subpopulations in human breast cancer tissue and its significance: an immunoperoxidase study with anti-human T- and B-cell sera.Cancer 1982;49:1456–64.
Wintzer H, Bohle W, von Kleist S. Study of the relationship between immunohistologically demonstrated lymphocytes infiltrating human breast carcinomas and patients' survival.Can Res Clin Oncol 1991;117:163–7.
Whitford P, Mallon EA, George WD, Campbell AM. Flow cytometric analysis of tumor infiltrating lymphocytes in breast cancer.Br J Can 1990;62:971–5.
Schwartzentruber DJ, Solomon D, Rosenberg SA, Topalian SL. Characterization of lymphocytes infiltrating human breast cancer: specific immune reactivity detected by measuring cytokine secretion.J Immunother 1992;12:1–12.
Miescher S, Whiteside TL, Carrel S, von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes.J Immunol 1986;136:1899–907.
Miescher S, Stoeck M, Qiao L, Barras C, Barrelet L, von Fliedner V. Preferential clonogenic deficit of CD8-positive T-lymphocytes infiltrating human solid tumors.Can Res 1988;48:6992–8.
Vose BM, Moore M. Supressor cell activity of lymphocytes infiltrating human lung and breast tumours.Int J Cancer 1979;24:579–85.
Asher AL, Mulé JJ, Kasid A, et al. Murine tumor cells transduced with the gene for tumor necrosis factor-α: evidence for paracrine immune effects of tumor necrosis factor against tumors.J Immunol 1991;146:3227–34.
Gansbacher B, Bannerji R, Daniels B, Zier K, Cronin K, Gilboa E. Retroviral vector-mediated γ interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity.Can Res 1990;50:7820–5.
Fearon ER, Pardoll DM, Itaya T, et al. Interleukin 2 production by tumor cells bypasses T helper function in the generation of an antitumor response.Cell 1990;60:397–403.
Dranoff G, Jaffel E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine GM-CSF stimulates potent, specific, and long lasting anti-tumor immunity.Proc Natl Acad Sci U S A 1993;90:3539–43.
Barth RJ, Mulé JJ, Spiess P, Rosenberg SA. Interferon γ and tumor necrosis factor have a role in tumor regressions mediated by murine tumor infiltrating lymphocytes.J Exp Med 1991;173:647–58.
Pisa P, Halapi E, Pisa EK, et al. Selective expression of interleukin 10, interferon γ, and granulocyte-macrophage colony-stimulating factor in ovarian cancer biopsies.Proc Natl Acad Sci U S A 1992;89:7708–12.
deWaal Malefyt R, Yssel H, deVries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells.J Immunol 1993;150:4754–65.
Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells.J Immunol 1994;153:811–6.
Schlaak JF, Hermann E, Gallati H, Zum Buschenfelde KH, Fleischer B. Differential effects of IL-10 on proliferation and cytokine production of human γ/δ and α/β T cells.Scand J Immunol 1994;39:209–15.
deWaal Malefyt R, Abrams JS, Bennet BJ, Figdor CG, de Vries JE. IL-10 inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes.J Exp Med 1991;174:1209–20.
Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profile of functional subsets of human CD4 and CD8 T cell clones.Science 1991;254:279–82.
Martinez O, Gibbons R, Garavoy FJ, Aronson FR. IL-4 inhibits IL-2 receptor expression and IL-2 dependent proliferation on human T cells.J Immunol 1990;144:2211–5.
Chen Y, Kuchroo V, Inobe J, Hafler D, Weiner H. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis.Science 1994;265:1237–40.
Kehrl JH, Wakefield LM, Roberts AB, et al. Production of transforming growth factor B by human T lymphocytes and its potential role in the regulation of T cell growth.J Exp Med 1986;163:1037–50.
Elston CW, Gresham GA, Rao GS, et al. The cancer research campaign (King's/Cambridge) trial for early breast cancer: clinico-pathological aspects.Br J Cancer 1982;45:655–69.
Dawson PJ, Karrsion T, Ferguson DJ. Histologic features associated with long term survival in breast cancer.Hum Pathol 1986;17:1015–21.
Rosen PP, Saigo PE, Braun DW, Weathers E, DePalo A. Predictors of recurrence in stage I (T1NOMO) breast carcinoma.Ann Surg 1981;193:15–25.
Tanaka M, Tanaka H, Ishikawa E. Immunohistochemical demonstration of surface antigen of human lymphocytes with monoclonal antibody in acetone-fixed paraffin-embedded sections.J Histochem Cytochem 1984;32:452–4.
Sato Y, Mukai K, Watanabe S, Goto M, Shimosato Y. The AMeX method: a simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining.Am J Pathol 1986;125:431–5.
Hoefakker S, van t Erve EHM, Deen C, et al. Immunohistochemical detection of co-localizing cytokine and antibody producing cells in the extrafollicular area of human palatine tonsils.Clin Exp Immunol 1993;93:223–8.
Pickvance EA, Oegema TR, Thompson RC. Immunolocalization of selected cytokines and proteases in canine articular cartilage after transarticular loading.J Orthop Res 1992;11:313–23.
Vitolo D, Zerbe T, Kanbour A, Dahl C, Herberman RB, Whiteside TL. Expression of mRNA for cytokines in tumor-infiltrating mononuclear cells in ovarian adenocarcinoma and invasive breast cancer.Int J Cancer 1992;51:573–80.
Schoof DD, Terashima Y, Peoples GP, et al. CD4+ T cell clones isolated from human renal cell carcinoma possess the functional characteristics of Th2 helper cells.Cell Immun 1993;150:114–23.
Whiteside TL, Miescher S, Hurlimann J, Moretta L, von Fliedner. Clonal analysis and in situ characterization of lymphocytes infiltrating human breast carcinomas.Can Immun Immunother 1986;23:169–78.
Alexander JP, Kudoh S, Melsop KA, et al. T cells infiltrating renal cell carcinoma display a poor proliferative response even though they can produce interleukin 2 and express interleukin 2 receptors.Can Res 1993;53:1380–7.
Finke J, Zea A, Stanley J, et al. Loss of T cell receptor z chain and p56lck in T cells infiltrating human renal cell carcinoma.Can Res 1993;53:5613–6.
Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro.Science 1994;264:1587–9.
McCume BK, Mullin BR, Flanders KC, Jaffurs WJ, Mullen LT, Sporn MB. Localization of transforming growth factor-beta isotypes in lesions of the human breast.Hum Pathol 1992;23:13–20.
Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. Immunohistochemical staining for transforming growth factor b1 associates with disease progression in human breast cancer.Can Res 1992;52:6949–52.
Walker RA, Dearing SJ, Gallacher B. Relationship of transforming growth factor beta 1 to extracellular matrix and stromal infiltrates in invasive breast carcinoma.Br J Cancer 1994;69:1160–5.
Murray PA, Barrett-Lee P, Travers M, Luqmani Y, Powles T, Coombes RC. The prognostic significance of transforming growth factors in human breast cancer.Br J Cancer 1993;67:1408–12.
Hwu P, Yannelli J, Kriegler M, et al. Functional and molecular characterization of tumor-infiltrating lymphocytes transduced with TNF-α cDNA for the gene therapy of cancer in humans.J Immunol 1993;150:4104–15.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Camp, B.J., Dyhrman, S.T., Memoli, V.A. et al. In situ cytokine production by breast cancer tumor-infiltrating lymphocytes. Annals of Surgical Oncology 3, 176–184 (1996). https://doi.org/10.1007/BF02305798
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02305798