Abstract
Aims/hypothesis
It is not known whether mean blood glucose (MBG) predicts the risk of macrovascular complications in diabetes any differently from HbA1c. In this study we therefore analysed data from the Diabetes Control and Complications Trial (DCCT) to assess the relationship between MBG, HbA1c and glucose variability with regard to the risk of cardiovascular disease in patients with type 1 diabetes.
Methods
Pre- and postprandial seven-point glucose profiles were collected quarterly during the DCCT in 1441 individuals. The relationship between time to first cardiovascular event and MBG, HbA1c and daily SD of blood glucose was assessed by Cox regression after adjusting for the known risk factors of macrovascular disease and the treatment groups of the patients.
Results
Cox regression showed MBG to be predictive of a cardiovascular event (p = 0.019), but not HbA1c (p = 0.858). A rise of 1 mmol/l in MBG was associated with an 11% rise in cardiovascular risk. MBG remained highly predictive (p = 0.015) even after adjustment for HbA1c values and glucose variability.
Conclusions/interpretation
This study has shown that during the DCCT MBG was a better predictor of the macrovascular complications of type 1 diabetes than HbA1c. It indicates that the cardiovascular risk associated with hyperglycaemia appeared within the time period of the study and that blood glucose rather than HbA1c may be the preferred means of assessing this risk.
Similar content being viewed by others
Introduction
Cardiovascular disease remains the main cause of excess mortality among patients with type 1 and type 2 diabetes [1–3]. While the Diabetes Control and Complications Trial (DCCT) in type 1 diabetes and the United Kingdom Prospective Diabetes Study (UKPDS) in type 2 diabetes both demonstrated the benefits of intensive glucose treatment with regard to the risk of microvascular complications [4, 5], the trends in both studies towards reduced cardiovascular events failed to reach statistical significance. However, post hoc analysis combining both groups in the UKPDS did seem to indicate that a 1% reduction in HbA1c was associated with a 14% fall in the risk of myocardial infarction, based on 573 events [6]. This was consistent with evidence emerging in type 1 diabetic patients [7] and participants without known diabetes [8], but was smaller in magnitude than the 37% reduction in microvascular complications per 1% HbA1c in the UKPDS [6] and the 44% reduction found in the DCCT for every 10% relative change (e.g. 7.2 vs 8.0%) in HbA1c [9]. More recently, the long-term follow-up study of the DCCT cohort, the Epidemiology of Diabetes Interventions and Complications (EDIC) Study, has found that intensive treatment during just the period of the DCCT reduced the long-term risk of cardiovascular disease by 42% [10]. Differences in HbA1c during the DCCT (rather than simply changes in known cardiovascular risk factors) seemed to account for much of the benefit associated with intensive treatment.
Although HbA1c is therefore an apparently useful marker for determining cardiovascular risk, it was originally used in the DCCT as an additional means of assessing glycaemia [11], as it gives an indication of glucose control during the 6 to 8 weeks prior to sampling [12]. In fact, blood glucose was also closely measured in the study, with each of the 1,441 participants requested to have 1 day glucose profiles (including pre- and postprandial measurements) performed every 3 months by a laboratory. This enabled a subsequent analysis to show that while there is a linear relationship between mean (AUC) blood glucose (MBG) and HbA1c, there can also be a marked discrepancy between the two measures with patients having the same MBG but quite different HbA1c values and vice versa [13]. By comparison, the UKPDS only collected fasting glucose measurements and so did not allow a similar analysis to be made.
We and other authors have previously shown that blood glucose variability around the mean value does not appear to be an additional factor in the development of microvascular complications among DCCT patients and that pre- and postprandial glucose values are equally predictive of the small vessel complications of type 1 diabetes [14, 15]. However, in patients with diabetes there is also now accumulating circumstantial evidence that glycaemic variability may well influence the risk of macrovascular complications, possibly through a mechanism mediated by free radicals [16].
No study has assessed the relative merits of MBG and HbA1c in predicting the risk of ischaemic heart disease and other macrovascular complications in patients with diabetes. We therefore re-analysed the original DCCT data to establish whether MBG or HbA1c was more predictive of these events independently of other known risk factors in patients with type 1 diabetes. We also wished to establish whether blood glucose variability exerted an additional influence on cardiovascular risk in these patients.
Methods
The datasets
We used the publicly accessible datasets collected by the DCCT, which were stored in SAS format (http://www.gcrc.umn.edu/gcrc/downloads.html). The DCCT was a 9 year randomised controlled trial of 1441 participants with type 1 diabetes that compared the effect of intensive vs conventional blood glucose management on the development of microvascular complications of diabetes. At randomisation, patients were stratified into one of two cohorts. The primary prevention cohort (n = 726) had no evidence of retinopathy by fundus photography and a urinary AER <40 mg/24 h (28 μg/min). The secondary prevention cohort (n = 715) had only minimal retinopathy and an AER <200 mg/24 h (140 μg/min). The study participants were randomised into intensive (n = 711) and conventional (n = 730) treatment groups.
Definition of events
Macrovascular events during the trial were those as defined by the DCCT group investigators and included angina, fatal and non-fatal myocardial infarction, coronary revascularisation and major ECG events [17]. Definitions, verification of macrovascular events and grading of electrocardiograms were standardised. Baseline characteristics and other data for patients who did or did not subsequently develop macrovascular disease have been presented previously [17].
Glycaemic variables and statistical methods
A capillary blood glucose profile was taken at intervals of 3 months with samples being analysed by a central laboratory [18]. Blood glucose was assessed at seven points throughout the day, namely: pre-breakfast (we assumed a time of 07:00 hours), post-breakfast (08:30 hours), pre-lunch (12:00 hours), post-lunch (13:30 hours), pre-supper (18:00 hours), post-supper (19:30 hours) and bedtime (22:00 hours). An additional data point was collected at 03:00 hours, but since this was only measured in <1% of participants, it is not considered further. MBG was calculated by the AUC using the trapezoidal rule [19] in accordance with Rohlfing et al. [13].
Instability of blood glucose (within-day SD) was calculated as the SD of daily blood glucose around the mean from each quarterly visit. This has been shown to correlate closely with other measures of glucose variability in type 1 diabetic patients [20]. Variability in MBG over time was estimated as the SD of the MBG (AUC) measurements measured every 3 months. Mean preprandial glucose was taken as the average of pre-breakfast, pre-lunch and pre-supper; mean postprandial glucose was taken as the average of post-breakfast, post-lunch and post-supper.
The relationship between time to first cardiovascular/macrovascular event and MBG (AUC), SD MBG, preprandial blood glucose, postprandial blood glucose and HbA1c was assessed by Cox regression, from which hazard ratios (HRs) and 95% CIs were calculated. Macrovascular event analysis included all the cardiovascular ones. Each of the variables was measured quarterly throughout the study period. We fitted a series of time-dependent Cox models that take into account the different measurements over time, to the data. In effect, the means are updated continuously. All Cox models were adjusted for the following baseline covariates: age (years), sex, disease duration (years), randomisation treatment (conventional vs intensive), prevention cohort (primary vs secondary), study phase (first or second) and BMI (kg/m2). Further statistical adjustment for a number of time-dependent variables (measured annually) was carried out, namely HDL-cholesterol (mmol/l), LDL-cholesterol (mmol/l), triacylglycerol (mmol/l), mean blood pressure (MBP; mmHg) and pack-years smoking. The GLIM4 [21] and SPSS (version 11) statistical computer packages were used to analyse the data. An arbitrary level of 5% statistical significance (two-tailed) was assumed.
Data considerations
A total of 1440 patients were included in the Cox modelling. One patient was excluded as his/her censoring time occurred before the earliest event in a stratum.
The DCCT remains the most complete randomised control trial in its field with a remarkably low loss to follow-up and outstanding compliance to the study protocol. Only eight patients withdrew, and 11 died. More than 95% of expected visits were completed. There were no missing data for the baseline covariates (age, sex, randomisation treatment, disease duration, study phase and prevention cohort).
With regard to time-dependent covariates, not everybody had a complete blood glucose profile during every quarter of their participation. Table 1 shows the compliance of patients throughout the study period. We included all profiles with five observations or more during the 24 h period, extrapolating missing values from the surrounding points. We are aware that many methods can be used to extrapolate missing values for longitudinal data [22] and that each method has its advantages and disadvantages depending on the setting [23]. Table 1 also describes the completeness of the other time-dependent covariates, most of which were collected annually. Missing data were not imputed, but were taken into account in the time-dependent modelling. Thus, if MBP was recorded at baseline but not at 1 year, the baseline value was used; if MBP was measured at 1 year but not at 2 years, the 1 year value was used and so on. Thus, the mean was constantly updated, replacing missing values with the last (most recent) observation carried forward until a new observation was available. Although we cannot rule out bias in this approach, the fact that this was the largest and most comprehensive diabetes study ever conducted [24] gives us confidence in our findings.
Results
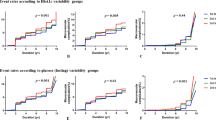
As already published by the DCCT group [17], 34 first cardiovascular events occurred during the trial, namely: five angina pectoris, eight cardiac arrhythmia, seven major ECG abnormalities, four definite myocardial infarction, four silent myocardial infarction, three deaths, two congestive heart failure and one coronary artery bypass grafting. A total of 73 other first macrovascular events also occurred: two cerebrovascular, 43 claudication, 25 persistent pedal pulse loss, two arterial events requiring surgery, two transient ischaemic attacks and one lower limb amputation. Cox regression models for cardiovascular and macrovascular risk (for first events), adjusted as described above, are presented in Table 2. Looked at individually, MBG (AUC), and pre- and postprandial blood glucose were significantly related to the development of cardiovascular risk, while within-day variability of MBG and HbA1c were not significant. When considered in a series of further models, MBG (AUC) was still associated with an increased risk of cardiovascular disease, even when adjustments were made for HbA1c values. While pre- and postprandial blood glucose were independently predictive of cardiovascular outcome, neither was significantly superior to the other when both were considered together. Relationships with any first macrovascular event (which include cardiovascular) were less strong all round than for cardiovascular events alone (Table 2). Similar findings were found when restricting analysis to only those glucose profiles with a full seven points collected on 75% or more of quarterly visits. For example, the HR for a cardiovascular event and MBG (as in model 2, Table 2) was 1.148 (95% CI = 1.036–1.272, p = 0.008), while for HbA1c the HR was 0.904 (95% CI = 0.717–1.138, p = 0.389). The HR for any macrovascular event and MBG was 1.124 (95% CI 1.032–1.234, p = 0.007) and for HbA1c 0.874 (95% CI = 0.715–1.068, p = 0.188).
Discussion
This study has shown that MBG, in contrast to HbA1c, was significantly predictive of cardiovascular events during the original study period of the DCCT following adjustment for the known risk factors of macrovascular disease and the treatment groups of the patients. For every 1 mmol/l increase in blood glucose the risk of a coronary event increased by 11% (p = 0.019). Indeed, MBG lost none of its ability to predict risk even when the HbA1c values of the study participants were taken into account. The reduced strength of association with MBG when other macrovascular diseases were included in the data analysis (p = 0.041) may be a reflection of the ‘softer’ nature of some of these endpoints as defined originally by the DCCT trialists.
Previous studies examining cardiovascular risk in the original DCCT dataset have concentrated mainly on differences between the treatment groups and have found trends but no statistically significant differences in event rate [4, 17]. The EDIC study group recently reported a 42% reduction in cardiovascular events among intensively treated DCCT patients during longer term follow-up, even though the glycaemia in intensive and non-intensive treatment groups converged after the end of the original trial [10]. This current analysis suggests that the risk associated with hyperglycaemia, which was found at 17 years follow-up by the EDIC study, was already largely in place during the original 6.5 years average follow-up in the DCCT.
This analysis also examined blood glucose variability during the DCCT, as well as pre- and postprandial measurements. Consistent with the lack of influence of glucose variability on microvascular complications [14], this analysis found no obvious evidence to corroborate a link with macrovascular risk in DCCT participants. Also, postprandial hyperglycaemia did not add to preprandial as a risk predictor (Table 2, model 3), but, as discussed below, these findings must be qualified because of the relatively few events in the study.
From a clinical perspective, this study reaffirms the usefulness of pre- and post-meal blood glucose measurement as an adjunct to HbA1c, especially in patients whose MBG is not entirely concordant with their HbA1c value. In this regard, an independent analysis of the DCCT dataset showed that individual patients with an MBG of, say, 10 mmol/l can have mean HbA1c values between 6 and 10% [13]. The reasons for these differences are multifactorial but are likely to include between-individual variability in erythrocyte survival, differences in the rate at which participants glycate their haemoglobin at the same glucose concentration [25] and variability in the relationship between mean glucose and HbA1c, depending on the glycaemic control of the population being studied [26]. Nonetheless, why MBG should be a better predictor than HbA1c of time to first cardiovascular event in the DCCT remains uncertain. It is, however, just possible that markers of hyperglycation (such as HbA1c) are superior at predicting the microvascular complications of diabetes, while hyperglycaemia is better for assessing cardiovascular risk.
This analysis is not without its limitations. There were only 34 first cardiovascular events and 73 other macrovascular events. This meant that there were therefore too few events in the intensive group to ascertain whether the findings applied equally to each treatment group. Also, while we did not find HbA1c to be a significant predictor of cardiovascular events in this study, it remains possible that we simply had insufficient power to detect smaller effects. To investigate this, we performed a post-hoc power calculation to determine the magnitude of HR that we could expect to detect in a study of this size [27]. Using an event rate of 2.4%, a variance of the covariate HbA1c of 1%, a sample size of 1441 and an alpha level of 0.05, we have sufficient (80%) power to detect an HR of 1.82 univariately. In comparison, the adjusted HR we observed for MBG was 1.11 and the adjusted HR observed for HbA1c was 1.03. We cannot conclude from the DCCT data that HbA1c will never predict cardiovascular risk (indeed it did after longer term follow-up in the EDIC study [10]), just that MBG seems to be a better predictor.
It must also be accepted that even though compliance with glucose profiling was very good for a clinical study of such length (Table 1), continuous glucose monitoring systems would probably prove to be a more accurate way of assessing MBG in future studies [28], even though it may also prove difficult to find another cohort as free from potential confounders, such as lipid-lowering, antiplatelet and antihypertensive treatments, as was present in the DCCT. There are also undoubted practical considerations if any form of glucose monitoring is to be recommended in addition to that of HbA1c measurement.
In summary, this study has shown that MBG was better than HbA1c at predicting the risk of cardiovascular disease in type 1 diabetic patients participating in the DCCT. High blood glucose values would thus appear to have exerted a detrimental effect on macrovascular complication risk even during the original duration of the DCCT study, despite this effect not being detected by measurement of HbA1c. In keeping with recommendations to reduce the microvascular complications of diabetes [29], this is an additional reason for controlling hyperglycaemia as rapidly as possible.
Abbreviations
- EDIC:
-
Epidemiology of Diabetes Interventions and Complications
- HR:
-
hazard ratio
- MBG:
-
mean blood glucose
- MBP:
-
mean blood pressure
- UKPDS:
-
United Kingdom Prospective Diabetes Study
References
Laing SP, Swerdlow AJ, Slater SD et al (2003) Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46:760–765
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234
Dorman JS, Laporte RE, Kuller LH et al (1984) The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes 33:271–276
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Stratton IM, Adler AI, Neil HA et al (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321:405–412
Lehto S, Ronnemaa T, Pyorala K, Laakso M (1999) Poor glycemic control predicts coronary heart disease events in patients with type 1 diabetes without nephropathy. Arterioscler Thromb Vasc Biol 19:1014–1019
Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N (2004) Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med 141:413–420
The Diabetes Control and Complications Trial Research Group (1995) The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 44:968–983
Nathan DM, Cleary PA, Backlund JY et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653
The Diabetes Control and Complications Trial Research Group (1987) Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabetes Care 10:1–19
Goldstein DE, Little RR, Wiedmeyer HM, England JD, McKenzie EM (1986) Glycated hemoglobin: methodologies and clinical applications. Clin Chem 32:B64–B70
Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE (2002) Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 25:275–278
Kilpatrick ES, Rigby AS, Atkin SL (2006) The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care 29:1486–1490
Service FJ, O’Brien PC (2001) The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia 44:1215–1220
Monnier L, Mas E, Ginet C et al (2006) Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295:1681–1687
The Diabetes Control and Complications Trial Research Group (1995) Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 75:894–903
The Diabetes Control and Complications Trial Research Group (1986) The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 35:530–545
Matthews JN, Altman DG, Campbell MJ, Royston P (1990) Analysis of serial measurements in medical research. BMJ 300:230–235
Moberg E, Kollind M, Lins PE, Adamson U (1993) Estimation of blood-glucose variability in patients with insulin-dependent diabetes mellitus. Scand J Clin Lab Invest 53:507–514
Francis B, Green M, Payne M (1994) The GLIM System. Release 4. Clarendon, Oxford
Diggle PH, Heagerty P, Liang K-Y, Zeger SL (2002) Analysis of longitudinal data. Oxford University Press, Oxford
Cozzi Lepri A, Smith G, Morcroft A, Sabin C, Morris R, Philips A (1998) A practical approach to adjusting for attrition bias in HIV clinical trials with serial marker responses. AIDS 12:1155–1161
Diabetes Control and Complications Trial (DCCT). Available from http://diabetes.niddk.nih.gov/dm/pubs/control/, accessed 22 August 2007
Yudkin JS, Forrest RD, Jackson CA, Ryle AJ, Davie S, Gould BJ (1990) Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Diabetologia 33:208–215
Kilpatrick ES, Rigby AS, Atkin SL (2007) Variability in the relationship between mean plasma glucose and HbA1c: implications for the assessment of glycemic control. Clin Chem 53:897–901
Hsieh F, Lavori P (2000) Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials 21:552–560
Klonoff DC (2005) Continuous glucose monitoring: Roadmap for 21st century diabetes therapy. Diabetes Care 28:1231–1239
American Diabetes Association (2007) Standards of medical care in diabetes—2007. Diabetes Care 30:S4–S41
Acknowledgements
We wish to thank the DCCT investigators for making their trial dataset public and therefore allowing independent investigators to analyse their work for the benefit of patients with type 1 diabetes.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kilpatrick, E.S., Rigby, A.S. & Atkin, S.L. Mean blood glucose compared with HbA1c in the prediction of cardiovascular disease in patients with type 1 diabetes. Diabetologia 51, 365–371 (2008). https://doi.org/10.1007/s00125-007-0883-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0883-x