Abstract
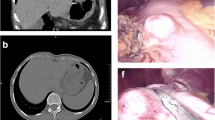
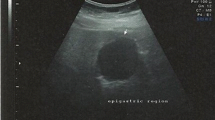
The frequency and morphological spectrum of gastrointestinal peripheral nerve sheath tumors (PNSTs) from consecutive case material has not been studied in the c-KIT era. We reviewed all mesenchymal gastrointestinal (GI) lesions at our departments according to current diagnostic criteria. PNSTs formed the third commonest group of mesenchymal GI tumors with a lower frequency (≤5%) compared to gastrointestinal stromal tumors (GISTs; ∼50%) and smooth muscle neoplasms (∼30%). Granular cell tumors (GCTs; n = 31) and schwannomas (n = 22) were the most common types of PNSTs encountered. Rare tumors included neurofibromatosis 1 (NF1)-associated PNSTs (n = 5) and gastric perineurioma (n = 1). Thirteen schwannomas (including also some recent cases) were initially diagnosed as GIST, leiomyoma, or neurofibroma. Unusual histological variants included sigmoid GCT with prominent lipomatous component (n = 1), reticular–microcystic schwannoma of small (n = 1) and large (n = 1) bowel, NF1-associated gastric schwannoma (the first case to date), and psammomatous melanotic colonic schwannoma unrelated to Carney complex (n = 1). PNSTs coexisted with GIST in four patients (three had definite NF1). In conclusion, PNSTs of the GI tract are rare uniformly benign neoplasms that may show schwannian, perineurial, fibroblastic, or mixed differentiation. Most of them (92%) occurred sporadically unassociated with NF1 or NF2. Gastrointestinal PNSTs are still underrecognized by general pathologists. Awareness of their diverse morphology will help to avoid confusing them with smooth muscle neoplasms and GIST that they may closely mimic.




Similar content being viewed by others
References
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23:70–83
Yagihashi S, Kimura M, Kurotaki H et al (1987) Gastric submucosal tumours of neurogenic origin with neuroaxonal and Schwann cell elements. J Pathol 153:41–50
Daimaru Y, Kido H, Hashimoto H et al (1988) Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol 19:257–264
Sarlomo-Rikala M, Miettinen M (1995) Gastric schwannoma—a clinicopathological analysis of six cases. Histopathology 27:355–360
Miettinen M, Shekitka KM, Sobin LH (2001) Schwannomas in the colon and rectum: a clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol 25:846–855
Kwon MS, Lee SS, Ahn GH (2002) Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract 198:605–613
Goh BK, Chow PK, Kesavan S et al (2008) Intraabdominal schwannomas: a single institution experience. J Gastrointest Surg 12:756–760
Hou YY, Tan YS, Xu JF et al (2006) Schwannoma of the gastrointestinal tract: a clinicopathological, immunohistochemical and ultrastructural study of 33 cases. Histopathology 48:536–545
Chetty R, Vajpeyi R, Penwick JL (2007) Psammomatous melanotic schwannoma presenting as colonic polyps. Virchows Arch 451:717–720
Hornick JL, Bundock EA, Fletcher CD (2009) Hybrid schwannoma/perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol 33:1554–1561
Scheithauer BW, Woodruff JM, Erlandson RA (1999) Tumors of the peripheral nervous system. In: Rosai J, Sobin LH (eds) Atlas of tumor pathology, 3rd series, fascicle 24. Armed Forces Institute of Pathology, Washington DC
Finkel G, Lane B (1982) Granular cell variant of neurofibromatosis: ultrastructure of benign and malignant tumors. Hum Pathol 13:959–963
Zarineh A, Costa ME, Rabkin MS (2008) Multiple hybrid granular cell tumor-perineuriomas. Am J Surg Pathol 32:1572–1577
Johnston MJ, Helwig EB (1981) Granular cell tumors of the gastrointestinal tract and perianal region: a study of 74 cases. Dig Dis Sci 26:807–816
Parfitt JR, McLean CA, Joseph MG et al (2006) Granular cell tumours of the gastrointestinal tract: expression of nestin and clinicopathological evaluation of 11 patients. Histopathology 48:424–430
Prematilleke IV, Sujendran V, Warren BF et al (2004) Granular cell tumour of the oesophagus mimicking a gastrointestinal stromal tumour on frozen section. Histopathology 44:502–503
Maiorano E, Favia G, Napoli A et al (2000) Cellular heterogeneity of granular cell tumours: a clue to their nature? J Oral Pathol & Med 29:284–290
Fine SW, Li M (2003) Expression of calretinin and the alpha-subunit of inhibin in granular cell tumors. Am J Clin Pathol 119:259–264
Mori T, Orikasa H, Shigematsu T et al (2006) An Ultrastructural and immunohistochemical study of a combined submucosal granular cell tumor and lipoma of the colon showing a unique nodule-in-nodule structure: putative implication of CD34 or prominin-2-positive stromal cells in its histopathogenesis. Virchows Arch 449:137–139
Plaza JA, Wakely PE Jr, Suster S (2006) Lipoblastic nerve sheath tumors: report of a distinctive variant of neural soft tissue neoplasm with adipocytic differentiation. Am J Surg Pathol 30:337–344
Hong R, Lim SC (2009) Granular cell tumor of the cecum with extensive hyalinization and calcification: a case report. World J Gastroenterol 15:3315–3318
Lee KH, Cho JH, Han YW et al (2006) A rare case of ossifying granular cell (Abrikossoff) tumour. Acta Derm Venereol 86:548–549
Adamiak A, Lee CH, Nielsen TO et al (2009) Duodenal epithelioid gastrointestinal stromal tumor with prominent granular cell features. Hum Pathol 40:599–602
Hirasaki S, Kanzaki H, Fujita K et al (2008) Ileal schwannoma developing into ileocolic intussusception. World J Gastroenterol 14:638–640
Jung MK, Jeon SW, Cho CM et al (2008) Gastric schwannomas: endosonographic characteristics. Abdom Imaging 33:388–390
Miettinen M, Wang ZF, Lasota J (2009) DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol 33:1401–1408
Liegl B, Bennett MW, Fletcher CD (2008) Microcystic/reticular schwannoma: a distinct variant with predilection for visceral locations. Am J Surg Pathol 32:1080–1087
Tozbikian G, Shen R, Suster S (2008) Signet ring cell gastric schwannoma: report of a new distinctive morphological variant. Ann Diagn Pathol 12:146–152
Fuller CE, Williams GT (1991) Gastrointestinal manifestations of type 1 neurofibromatosis (von Recklinghausen's disease). Histopathology 19:1–11
Basile U, Cavallaro G, Polistena A et al (2010) Gastrointestinal and retroperitoneal manifestations of type 1 neurofibromatosis. J Gastrointest Surg 14:186–194
Zámecník M, Mukensnabl P, Sokol L et al (2004) Perineurial cells and nerve axons in gastrointestinal schwannomas: a similarity with neurofibromas. An immunohistochemical study of eight cases. Cesk Patol 40:150–153
Lasota J, Wasag B, Dansonka-Mieszkowska A et al (2003) Evaluation of NF2 and NF1 tumor suppressor genes in distinctive gastrointestinal nerve sheath tumors traditionally diagnosed as benign schwannomas: a study of 20 cases. Lab Invest 83:1361–1371
Ogasawara N, Sasaki M, Ishiguro H et al (2009) Gastric schwannoma with adjacent external progression harbored aberrant NF2 gene. Dig Endosc 21:192–195
Fine SW, McClain SA, Li M (2004) Immunohistochemical staining for calretinin is useful for differentiating schwannomas from neurofibromas. Am J Clin Pathol 122:552–559
Ince AT, Yavuzer D, Kiliç G et al (2008) Coincidental occurrence of granular cell tumor and gastrointestinal stromal tumor in a patient. Turk J Gastroenterol 19:135–136
Agaimy A, Wünsch PH, Hofstaedter F et al (2007) Minute gastric sclerosing stromal tumors (GIST tumorlets) are common in adults and frequently show c-KIT mutations. Am J Surg Pathol 31:113–120
Hornick JL, Fletcher CD (2005) Soft tissue perineurioma: clinicopathologic analysis of 81 cases including those with atypical histologic features. Am J Surg Pathol 29:845–858
Agaimy A, Wünsch PH (2005) Perineurioma of the stomach: a rare spindle cell neoplasm that should be distinguished from gastrointestinal stromal tumor. Pathol Res Pract 201:463–467
Hornick JL, Fletcher CD (2005) Intestinal perineuriomas: clinicopathologic definition of a new anatomic subset in a series of 10 cases. Am J Surg Pathol 29:859–865
Kelesidis T, Tarbox A, Lopez M et al (2009) Perineurioma of esophagus: a first case report. Am J Med Sci 338:230–232
Emanuel P, Pertsemlidis DS, Gordon R et al (2006) Benign hybrid perineurioma-schwannoma in the colon. A case report. Ann Diagn Pathol 10:367–370
Groisman GM, Polak-Charcon S (2008) Fibroblastic polyp of the colon and colonic perineurioma: 2 names for a single entity? Am J Surg Pathol 32:1088–1094
Lewin MR, Dilworth HP, Abu Alfa AK et al (2005) Mucosal benign epithelioid nerve sheath tumors. Am J Surg Pathol 29:1310–1315
Gibson JA, Hornick JL (2009) Mucosal Schwann cell "hamartoma": clinicopathologic study of 26 neural colorectal polyps distinct from neurofibromas and mucosal neuromas. Am J Surg Pathol 33:781–787
Telem DA, Pertsemlidis D (2008) Malignant peripheral nerve sheath tumor: an unusual cause of intussusception. J Gastrointest Surg 12:1609–1611
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agaimy, A., Märkl, B., Kitz, J. et al. Peripheral nerve sheath tumors of the gastrointestinal tract: a multicenter study of 58 patients including NF1-associated gastric schwannoma and unusual morphologic variants. Virchows Arch 456, 411–422 (2010). https://doi.org/10.1007/s00428-010-0886-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-0886-8