Abstract
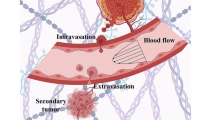
Cancer metastasis follows a sequential series of events, and many of the critical steps are distinctly similar to EMT-like transformations that occur during normal embryonic development. A current area of focus is the similarities between how cancer cells interact with the ectopic parenchyma after metastatic spread, and secondary developmental MET events that occur in epithelial tissues that have re-assembled within the embryo from mesenchymal cells. Accumulating evidence suggests a critical role for these secondary events, termed mesenchymal-epithelial transitions (MET) in development and mesenchymal-epithelial reverting transitions (MErT) in cancer. In this situation, metastatic seed cancer cells may inertly become part of the ectopic tissue and therefore surmount the metastatic inefficiencies to which most disseminated cancer cells succumb. Just as a critical EMT event is the downregulation or silencing of E-cadherin, we discuss the role of E-cadherin in cancer-associated MErT at distant metastatic sites and speculate on the implications for the fate of micrometastases that undergo a transition to being E-cadherin positive.





Similar content being viewed by others
References
Babu M, Wells A (2001) Dermal-epidermal communication in wound healing. Wounds 13:183–189
Birchmeier C, Birchmeier W, Gherardi E, VandeWoude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4:915–925
Boccaccio C, Comoglio PM (2006) Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer 6:637–645
Christiansen JJ, Rajasekaran AK (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 66:8319–8326
Christofori G (2006) New signals from the invasive front. Nature 441:444–450
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze’ev A (2003) Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of b-catenin signaling, Slug, and MAPK. J Cell Biol 163:847–857
Cristofanilli M, Budd GT et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781–791
Ewing J (1922) Tumors of the prostate. Neoplastic diseases. WB Saunders Company, Philadelphia, pp 784–785
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Gleave ME, Hsieh JT, vonEschenbach AC, Chung LWK (1992) Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol 147:1151–1159
Graff JR, Gabrielson E, Fujii H, Baylin SB, Herman JG (2000) Methylation patterns of the E-cadherin 5’CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J Biol Chem 275:2727–2732
Guarino M, Rubino B, Ballabio G (2007) The role of epithelial-mesenchymal transition in cancer pathology. Pathology 39:305–318
Gumbiner BM (2005) Regualtion of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6:622–634
Hazan RB, Norton L (1998) The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem 273:9078–9084
Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW (2007) Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol 213:374–383
Imai T, Horiuchi A et al (2004) Elevated expression of E-cadherin and alpha-, beta-, and gamma-catenins in metastatic lesions compared with primary epithelial ovarian carcinomas. Hum Pathol 35:1469–1476
Jones PA, Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3:415–428
Kaihara T, Kawamata H, Imura J, Fujii S, Kitajima K, Omotehara F, Maeda N, Nakamura T, Fujimori T (2003) Redifferentiation and ZO-1 reexpression in liver-metastatized colorectal cancer: possible association with epidermal growth factor receptor-induced tyrosine phosphorylation of ZO-1. Cancer Sci 94:166–172
Kalluri R, Neilson EG (2003) Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784
Kang H-G, Jenabi JM et al (2007) E-cadherin cell–cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res 67:3094–3105
Kang Y, Massague J (2004) Epithelial-mesenchymal transitions: twist in development and metastasis. Cell 118:277–279.
Kim H, Turner T, Kassis J, Souto J, Wells A (1999) EGF receptor signaling in prostate development. Histol Histopathol 14:1175–1182
Kopstein L, Christofori G (2006) Metastasis: cell autonomous mechanisms versus contributions by the tumor microenvironment. Cell Mol Life Sci 63:449–468
Kowalski PJ, Rubin MA, Kleer CG (2003) E-cadherin expression in primary carcinoma of the breast and its distant metastases. Breast Cancer Res 5:R217–R222
Luzzi KJ, MacDonald IC et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–873
Mamoune A, Kassis J et al (2004) DU145 human prostate carcinoma invasiveness is modulated by urokinase receptor (uPAR) downstream of epidermal growth factor receptor (EGFR) signaling. Exp Cell Res 299:91–100
Miura H, Nishimura K et al (2001) Effects of hepatocyte growth factor on E-cadherin-mediated cell–cell adhesion in DU145 prostate cancer cells. Urology 58:1064–1069
Moustakas A, Heldin C-H (2007) Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci 98:1512–1520
Nelson WJ, Nusse R (2004) Convergence of wnt, b-catenin, and cadherin pathways. Science 303:1483–1487
Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8:98–101
Putz E, Witter K et al (1999) Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res 59:241–248
Reddy P, Liu L et al (2005) Formation of E-cadherin mediated cell–cell adhesion activates Akt and mitogen activated protein kinase (MAPK) via phosphatidylinositol 3 kinase and ligand-independent action of epidermal growth factor (EGF) receptor in ovarian cancer cells. Mol Endocrinol 19:2564–2578
Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z (1994) Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14:8333–8342
Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML (2001) E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum Pathol 32:690–697
Shah RB, Mehra R et al (2004) Androgen-independent prostate cancer is a heterogeneous group of diseases. Cancer Res 64:9209–9216
Shepard CR, Yates CC, Wells A (2007) Signaling pathway activation upon re-expression of E-cadherin in invasive breast cancer cells and interaction with ectopic normal epithelial cells (unpublished data)
Simcha I, Geiger B, Yehuda-Levenberg S, Salomon D, Ben-Ze’ev A (1996) Suppression of tumorigenicity by plakoglobin: an augmenting effect of N-cadherin. J Cell Biol 133:199–209
Stessels F, VandenEynden G et al (2004) Breast adenocarcinoma liver metastases, in contrast to colorectal liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br J Cancer 90:1429–1436
Strathdee G (2002) Epigenetic versus genetic alterations in the inactivation of E-cadherin Seminars. Cancer Biol 12:373–379
Tarin D (2005) The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 65:5996–6000
Thompson EW, Newgreen DF (2005) Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition. Cancer Res 65:5991–5995
Wells A (2000) Tumor invasion: role of growth factor-induced cell motility. Adv Cancer Res 78:31–101
Yates C, Shepard CR et al (2007a) Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Adv Cancer Res 96:225–246
Yates C, Shepard CR, Stolz DB, Wells A (2007b) Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer 96:1246–1252
Yates CC, Shepard CR, Stolz DB, Wells A (2007c) Co-culturing human prostate carcinoma cells with hepatocytes leads to increased expression of E-cadherin. Br J Cancer 96:1246–1252
Yates C, Wells A, Turner T (2005) Luteinizing hormone releasing hormone (LHRH) analog reverses the cell adhesion profile of DU-145 human prostate carcinoma. Br J Cancer 92:366–375
Acknowledgements
The authors thank the support from the VA Merit Program, the DoD’s Congressionally Directed Medical Research Programs on Prostate and Breast Cancer, and the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wells, A., Yates, C. & Shepard, C.R. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis 25, 621–628 (2008). https://doi.org/10.1007/s10585-008-9167-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-008-9167-1