Abstract
Background
Common variable immunodeficiency (CVID) is a heterogeneous disorder characterized by recurrent infections and defective immunoglobulin production.
Methods
The DEFI French national prospective study investigated peripheral T-cell and B-cell compartments in 313 CVID patients grouped according to their clinical phenotype, using flow cytometry.
Results
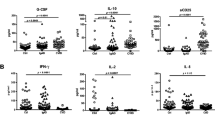
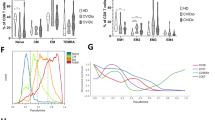
In patients developing infection only (IO), the main B-cell or T-cell abnormalities were a defect in switched memory B cells and a decrease in naive CD4+ T cells associated with an increase in CD4+CD95+ cells. These abnormalities were more pronounced in patients developing lymphoproliferation (LP), autoimmune cytopenia (AC), or chronic enteropathy (CE). Moreover, LP and AC patients presented an increase in CD21low B cells and CD4+HLA-DR+ T cells and a decrease in regulatory T cells.
Conclusion
In these large series of CVID patients, the major abnormalities of the B-cell and T-cell compartments, although a hallmark of CVID, were only observed in half of the IO patients and were more frequent and severe in patients with additional lymphoproliferative, autoimmune, and digestive complications.




Similar content being viewed by others
References
Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93:190–7.
Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–86.
Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27:308–16.
Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48.
Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993;86:31–42.
Piqueras B, Lavenu-Bombled C, Galicier L, Bergeron-van der Cruyssen F, Mouthon L, Chevret S, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23:385–400.
Warnatz K, Denz A, Drager R, Braun M, Groth C, Wolff-Vorbeck G, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(−)IgD(−)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99:1544–51.
Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111:77–85.
Giovannetti A, Pierdominici M, Mazzetta F, Marziali M, Renzi C, Mileo AM, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–43.
Guazzi V, Aiuti F, Mezzaroma I, Mazzetta F, Andolfi G, Mortellaro A, et al. Assessment of thymic output in common variable immunodeficiency patients by evaluation of T cell receptor excision circles. Clin Exp Immunol. 2002;129:346–53.
Di Renzo M, Serrano D, Zhou Z, George I, Becker K, Cunningham-Rundles C. Enhanced T cell apoptosis in common variable immunodeficiency: negative role of the fas/fas ligand system and of the Bcl-2 family proteins and possible role of TNF-RS. Clin Exp Immunol. 2001;125:117–22.
Aukrust P, Muller F, Froland SS. Elevated serum levels of interleukin-4 and interleukin-6 in patients with common variable immunodeficiency (CVI) are associated with chronic immune activation and low numbers of CD4+ lymphocytes. Clin Immunol Immunopathol. 1994;70:217–24.
Sneller MC, Strober W. Abnormalities of lymphokine gene expression in patients with common variable immunodeficiency. J Immunol. 1990;144:3762–9.
Kondratenko I, Amlot PL, Webster AD, Farrant J. Lack of specific antibody response in common variable immunodeficiency (CVID) associated with failure in production of antigen-specific memory T cells. MRC Immunodeficiency Group. Clin Exp Immunol. 1997;108:9–13.
Stagg AJ, Funauchi M, Knight SC, Webster AD, Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 1994;96:48–53.
Oliva A, Scala E, Quinti I, Paganelli R, Ansotegui IJ, Giovannetti A, et al. IL-10 production and CD40L expression in patients with common variable immunodeficiency. Scand J Immunol. 1997;46:86–90.
Farrington M, Grosmaire LS, Nonoyama S, Fischer SH, Hollenbaugh D, Ledbetter JA, et al. CD40 ligand expression is defective in a subset of patients with common variable immunodeficiency. Proc Natl Acad Sci USA. 1994;91:1099–103.
Paccani SR, Boncristiano M, Patrussi L, Ulivieri C, Wack A, Valensin S, et al. Defective Vav expression and impaired F-actin reorganization in a subset of patients with common variable immunodeficiency characterized by T-cell defects. Blood. 2005;106:626–34.
Boncristiano M, Majolini MB, D'Elios MM, Pacini S, Valensin S, Ulivieri C, et al. Defective recruitment and activation of ZAP-70 in common variable immunodeficiency patients with T cell defects. Eur J Immunol. 2000;30:2632–8.
Oksenhendler E, Gerard L, Fieschi C, Malphettes M, Mouillot G, Jaussaud R, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008;46:1547–54.
Malphettes M, Gerard L, Carmagnat M, Mouillot G, Vince N, Boutboul D, et al. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis. 2009;49:1329–38.
Fournier S, Rabian C, Alberti C, Carmagnat MV, Garin JF, Charron D, et al. Immune recovery under highly active antiretroviral therapy is associated with restoration of lymphocyte proliferation and interferon-gamma production in the presence of Toxoplasma gondii antigens. J Infect Dis. 2001;183:1586–91.
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300.
Mullighan CG, Fanning GC, Chapel HM, Welsh KI. TNF and lymphotoxin-alpha polymorphisms associated with common variable immunodeficiency: role in the pathogenesis of granulomatous disease. J Immunol. 1997;159:6236–41.
Mannon PJ, Fuss IJ, Dill S, Friend J, Groden C, Hornung R, et al. Excess IL-12 but not IL-23 accompanies the inflammatory bowel disease associated with common variable immunodeficiency. Gastroenterology. 2006;131:748–56.
Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117:289–300.
Fevang B, Yndestad A, Sandberg WJ, Holm AM, Muller F, Aukrust P, et al. Low numbers of regulatory T cells in common variable immunodeficiency: association with chronic inflammation in vivo. Clin Exp Immunol. 2007;147:521–5.
Genre J, Errante PR, Kokron CM, Toledo-Barros M, Camara NO, Rizzo LV. Reduced frequency of CD4(+)CD25(HIGH)FOXP3(+) cells and diminished FOXP3 expression in patients with common variable immunodeficiency: a link to autoimmunity? Clin Immunol. 2009;132:215–21.
Sanchez-Ramon S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol. 2008;128:314–21.
Alachkar H, Taubenheim N, Haeney MR, Durandy A, Arkwright PD. Memory switched B cell percentage and not serum immunoglobulin concentration is associated with clinical complications in children and adults with specific antibody deficiency and common variable immunodeficiency. Clin Immunol. 2006;120:310–8.
Ko J, Radigan L, Cunningham-Rundles C. Immune competence and switched memory B cells in common variable immunodeficiency. Clin Immunol. 2005;116:37–41.
Moratto D, Gulino AV, Fontana S, Mori L, Pirovano S, Soresina A, et al. Combined decrease of defined B and T cell subsets in a group of common variable immunodeficiency patients. Clin Immunol. 2006;121:203–14.
Livaditi O, Giamarellos-Bourboulis EJ, Kakkas I, Kapsimali V, Lymberi P, Papastariades C, et al. Grouping of patients with common variable immunodeficiency based on immunoglobulin biosynthesis: comparison with a classification system on CD4-naive cells. Immunol Lett. 2007;15:103–9.
Acknowledgments
This study was supported by a national program on rare diseases (GIS-Maladies Rares), by the National Program for Clinical Research (PHRC 2005), and by the National Center on Hereditary Immune Deficiencies (CEREDIH).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
A complete list of the DEFI Study Group is presented in Appendix A.
Appendix A. DEFI Study Group
Appendix A. DEFI Study Group
-
Coordination: Hôpital Saint Louis, Paris: E. Oksenhendler
-
Clinical Centers: Hôpital Saint Louis, Paris: C. Fieschi, M. Malphettes, L. Galicier, S. Georgin, J.P. Fermand. Bordeaux: J.F. Viallard. Limoges: A. Jaccard. Tours: C. Hoarau, Y. Lebranchu. Hôpital Cochin, Paris: A. Bérezné, L. Mouthon. HEGP, Paris: M. Karmochkine. Marseille: N. Schleinitz. Lyon Sud: I. Durieu, R. Nove-Josserand. Clermont-Ferrand: V. Chanet. Montpellier: V. Le-Moing. Roubaix: N. Just. Hôtel-Dieu, Paris: C. Salanoubat. Reims: R. Jaussaud. Hôpital Necker, Paris: F. Suarez, O. Hermine. Le Mans: P. Solal-Celigny. Lille: E. Hachulla. Perpignan: L. Sanhes. Angers: M. Gardembas, I. Pellier. Troyes: P. Tisserant. Lyon Armée: M. Pavic. Dijon: B. Bonnotte. Pitié-Salpêtrière, Paris: J. Haroche, Z. Amoura. Toulouse: L. Alric, M.F. Thiercelin, L. Tetu, D. Adoue. Nancy Vandoeuvre: P. Bordigoni. Lyon Croix Rousse: T. Perpoint. Lyon Hotel-Dieu: P. Sève. Besançon: P. Rohrlich. Strasbourg: J.L. Pasquali, P. Soulas. Hôpital Foch, Suresnes: L.J. Couderc. Montauban: P. Giraud. Hôpital Saint-Louis, Pédiatrie, Paris: A. Baruchel. Clermont-Ferrand 2: I. Deleveau. Kremlin-Bicêtre: F. Chaix. Hôpital Trousseau, Paris: J. Donadieu. Rouen: F. Tron. Bobigny: C. Larroche. Aix: A.P. Blanc. Nantes: A. Masseau, M. Hamidou. Nancy: G. Kanny, M. Morisset. Poitiers: F. Millot. Bondy: O. Fain. Hôpital Bichat, Paris: R. Borie. Rennes: A. Perlat
-
Laboratories: Pitié-Salpêtrière, INSERM U543, Paris: P. Debré, G. Mouillot, I. Théodorou. Saint-Louis, Immunologie, Paris: C. Rabian, M. Carmagnat. Saint-Louis, EA 3963, Paris: C. Fieschi, M. Malphettes, N. Vince, D. Boutboul
-
Clinical research assistants: A. De Gouvello, A. Gardeur.
-
Data management and statistics: L. Gérard.
Rights and permissions
About this article
Cite this article
Mouillot, G., Carmagnat, M., Gérard, L. et al. B-Cell and T-Cell Phenotypes in CVID Patients Correlate with the Clinical Phenotype of the Disease. J Clin Immunol 30, 746–755 (2010). https://doi.org/10.1007/s10875-010-9424-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-010-9424-3