Abstract
Purpose
Urinary free cortisol (UFC) determination by highly specific methods as mass spectrometry instead of commercially available antibody-based immunoassays is increasingly recommended. However, clinical comparisons of both analytical approaches in the screening of Cushing’s syndrome (CS) are not available. The aim of this study was to evaluate the diagnostic value of mass spectrometry versus immunoassay measurements of 24 h-UFC in the screening of CS.
Methods
Cross-sectional study of 33 histologically confirmed CS patients: 25 Cushing’s disease, 5 adrenal CS and 3 ectopic CS; 92 non-CS patients; and 35 healthy controls. UFC by immunoassay (UFCxIA) and mass spectrometry (UFCxMS), urinary free cortisone (UFCo) and UFC:UFCo ratio were measured, together with creatinine-corrected values. Sensitivity, specificity, AUC and Landis and Koch concordance index were determined.
Results
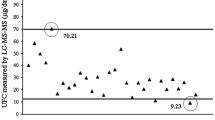
AUC for UFCxIA and UFCxMS were 0.77 (CI 0.68–0.87) and 0.77 (CI 0.67–0.87) respectively, with a kappa coefficient 0.60 and strong Landis and Koch concordance index. The best calculated cutoff values were 359 nmol/24 h for UFCxIA (78 % sensitivity, 62 % specificity) and 258.1 nmol/24 h for UCFxMS (53 % sensitivity, 86 % specificity). The upper limit of UFCxIA and UCFxMS reference ranges were 344.7 and 169.5 nmol/24 h respectively. Sensitivity and specificity for CS diagnosis at these cutpoints were 84 and 56 % for UFCxIA and 81 and 54 % for UFCxMS.
Conclusions
According to our data, both methods present a very similar diagnostic value. However, results suggest that lower cutoff points for mass spectrometry may be necessary in order to improve clinical sensitivity.


Similar content being viewed by others
References
Pivonello R, Faggiano A, Lombardi G, Colao A (2005) The metabolic syndrome and cardiovascular risk in Cushing’s syndrome. Endocrinol Metab Clin North Am 34:327–339
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367:1605–1617
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:1526–1540
Raff H, Sharma ST, Nieman LK (2014) Physiological basis for the etiology, diagnosis and treatment of adrenal disorders: cushing’s syndrome, adrenal insufficiency, and congenital hyperplasia. Compr Physiol 4:739–769
Raff H, Auchus R, Findling JW, Nieman LK (2015) Urine free cortisol in the diagnosis of Cushing’s syndrome: is it worth doing and if so, how? J Clin Endocrinol Metab 100:395–397
Rosmalen JG, Kerma IP, Wüst S, van der Ley C, Visser ST, Snieder H, Bakker SJ (2014) 24 h urinary free cortisol in large-scale epidemiological studies: short-term and long-term stability and sources of variability. Psychoneuroendocrinology 47:10–16
Fong BM, Tam S, Leung KS (2010) Improved liquid chromatography–tandem mass spectrometry method in clinical utility for the diagnosis of Cushing’s syndrome. Anal Bioanal Chem 396:783–790
Wood L, Ducroq DH, Fraser HL, Gillingwater S, Evans C, Pickett AJ, Rees DW, John R, Turkes A (2008) Measurement of urinary free cortisol by tandem mass spectrometry and comparison with results obtained by gas chromatography–mass spectrometry and two commercial immunoassays. Ann Clin Biochem 45:380–388
Handelsman DJ, Wartofsky L (2013) Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab 98:3971–3973
Aranda G, Enseñat J, Mora M, Puig-Domingo M, Martínez de Osaba MJ, Casals G, Verger E, Ribalta MT, Hanzu FA, Halperin I (2015) Long-term remission and recurrence rate in a cohort of Cushing’s disease: the need for long-term follow-up. Pituitary 18:142–149
Casals G, Marcos J, Pozo ÓJ, Aguilera P, Herrero C, To-Figueras J (2013) Gas chromatography-mass spectrometry profiling of steroids in urine of patients with acute intermittent porphyria. Clin Biochem 46:819–824
Monaghan PJ, Keevil BG, Trainer PJ (2013) Mass spectrometry for the endocrine clinic-much to digest. Clin Endocrinol (Oxf) 78:344–346
Turpeinene U, Hamalainen E (2013) Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab 27:795–801
Cecatto F, Antonelli G, Barbot M, Zilio M, Mazzai L, Gatti R, Zaninotto M, Mantero F, Boscaro M, Plebani M, Scaroni C (2014) The diagnostic performance of urinary free cortisol is better than the cortisol: cortisone ratio in detecting de novo Cushing’s syndrome: the use of a LC–MS/MS method in routine clinical practice. Eur J Endocrinol 171:1–7
Taylor A, Keevil B, Huhtaniemi I (2015) Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur J Endocrinol 173:D1–D12
Krone N, Hughes BA, Lavery GG, Stewart PM, Arlt W, Shackleton CH (2010) Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem Mol Biol 121:496–504
Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, Ennis R, Erwin PJ, Montori VM (2008) Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab 93:1553–1562
Carroll T, Raff H, Findling JW (2009) Late-night salivary cortisol for the diagnosis of Cushing syndrome: a meta-analysis. Endocr Pract 15:335–342
Ceccato F, Barbot M, Zilio M, Frigo AC, Albiger N, Camozzi V, Antonelli G, Plebani M, Mantero F, Boscaro M, Scaroni C (2015) Screening test for Cushing’s syndrome: urinary free cortisol role measured by LC–MS/MS. J Clin Endocrinol Metab 100:3856–386120
Cooper MS, Stewart PM (2009) 11β-Hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus–pituitary–adrenal axis, metabolic syndrome, and inflammation. J Clin Endocrinol Metab 94:4645–4654
Stiefel P, García-Morillo JS, Jimenez L, Pamies E, Miranda ML, Carneado J, Villar J, Leal-Cerro A (2002) Role of ketoconazole treatment in urinary free cortisol to cortisone and tetrahydrocortisol to tetrahydrocortisone ratios in non-ectopic Cushing syndrome. Endocrine 18:279–284
Alexandraki KI, Grossman AB (2010) The ectopic ACTH syndrome. Rev Endocr Metab Disord 11:117–126
Acknowledgments
The authors are indebted to Dr. María Jesús Martínez de Osaba for her expert advice and insights that inspired this work.
Funding
The study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
M. Careaga, F. A. Hanzu, I. Halperin and G. Casals have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Aranda, G., Careaga, M., Hanzu, F.A. et al. Accuracy of immunoassay and mass spectrometry urinary free cortisol in the diagnosis of Cushing’s syndrome. Pituitary 19, 496–502 (2016). https://doi.org/10.1007/s11102-016-0730-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-016-0730-5