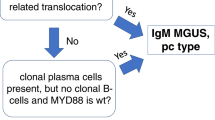
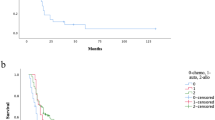
Abstract
Posttransplant lymphoproliferative disorder (PTLD) is a heterogeneous disease group of benign and malignant entities. The new World Health Organisation classification introduced in 2008 distinguishes early lesions, polymorphic, monomorphic and classical Hodgkin lymphoma-type PTLD. Based on the time of appearance, early and late forms can be identified.
PTLDs are the second most frequent posttransplantation tumors in adulthood, and the most frequent ones in childhood. The incidence varies with the transplanted organ—from 1%–2% following kidney transplantation to as high as 10% following thoracic organ transplantation—due to different intensities in immunosuppression. Immunocompromised state and Epstein-Barr virus (EBV) infection are the two major risk factors.
In Europe and the US approximately 85% of PTLDs are of B-cell origin, and the majority are EBV-associated. Symptoms are often unspecific; extranodal, organ manifestations and central nervous system involvement is common. Early lesions respond well to a decrease in immunosuppression. Malignant entities are treated with rituximab, chemotherapy, radiotherapy and surgical therapy. Adoptive T-cell transfer represents a promising therapeutic approach. The prognosis is favorable in early PTLD, and poor in late PTLD. Five-year survival is 30% for high-grade lymphomas. The prognosis of EBV-negative lymphomas is worse.
Lowering the risk of PTLD may be achieved by low dose maintenance immunosuppression, immunosuppressive drugs inhibiting cell proliferation, and special immunotherapy (e.g. interleukin-2 inhibitors). Early detection is especially important for high risk—e.g. EBV-negative—patients, where the appearance of EBV-DNA and the increase in its titer may help.


Similar content being viewed by others
Abbreviations
- ATG:
-
Anti-thymocyte globulin
- CMV:
-
Cytomegalovirus
- CNI:
-
Calcineurin inhibitor
- CNS:
-
Central nervous system
- CTL:
-
Cytotoxic T-lymphocyte
- DLBCL:
-
Diffuse large B-cell lymphoma
- DNA:
-
Deoxyribonucleic acid
- EBNA:
-
Epstein-Barr nuclear antigen
- EBV:
-
Epstein-Barr virus
- FDG-PET:
-
Fluorodeoxyglucose positron emission tomography
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- HSCT:
-
Hematopoietic stem cell transplantation
- HTLV:
-
Human T-cell leukemia virus
- IL:
-
Interleukin
- LMP:
-
Latent membrane protein
- mTOR:
-
Mammalian target of rapamycin
- NK:
-
Natural killer
- PCR:
-
Polymerase chain reaction
- PSI:
-
Proliferation signal inhibitor
- PTLD:
-
Posttransplant lymphoproliferative disorders
- SOT:
-
Solid organ transplantation
- UNOS:
-
United Network for Organ Sharing
- WHO:
-
World Health Organisation
References
Penn I (1999) Posttransplant malignancies. Transplant Proc 31:1260–1262
Vasudev B, Hariharan S (2007) Cancer after transplantation. Curr Opin Nephrol Hypertens 16:523–528
ANZDATA A, Zealand N (2006) Dialysis and Transplant Registry. ANZDATA Registry, Woodville
Kasiske BL, Snyder JJ, Gilbertson DT et al (2004) Cancer after kidney transplantation in the United States. Am J Transplant 4:905–913
Buell JF, Gross TG, Woodle ES (2005) Malignancy after transplantation. Transplantation 80:S254–S264
Doak PB, Montgomerie JZ, North JD et al (1968) Reticulum cell sarcoma after renal homotransplantation and azathioprine and prednisone therapy. Br Med J 4:746–748
Starzl TE (1968) Discussion of Murray JE, Wilson RE, Tilney NL et al Five years’ experience in renal transplantation with immunosuppressive drugs: survival, function, complications and the role of lymphocyte depletion by thoracic duct fistula. Ann Surg 168:416–435
Penn I, Starzl TE (1972) Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation 14:407
Penn I (1975) The incidence of malignancies in transplant recipients. Transplant Proc 7:323–326
Starzl TE, Nalesnik MA, Porter KA et al (1984) Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet 1(8377):583–587
Harris NL, Jaffe ES, Diebold J et al (2000) The World Health Organization classification of neoplastic diseases of the haematopoietic and lymphoid tissues: Report of the Clinical Advisory Committee Meeting. Airlie House, Virginia, November 1997. Histopathology 36:69–86
Harris NL, Swerdlow SH, Frizzera G et al (2001) Posttransplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds) World Health Organization classification of tumors. Pathology and genetics of tumors of haematopoietic and lymphoid tissue. IARC Press, Lyon, pp 264–269
Swerdlow SH, Webber SA, Chadburn A et al (2008) Post-transplant lymphoproliferative disorders (PTLD). In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. IARC Press, Lyon, pp 343–350
Mucha K, Foroncewicz B, Ziarkiewicz-Wróblewska B et al (2010) Post-transplant lymphoproliferative disorder in view of the new WHO classification: a more rational approach to a protean disease ? Nephrol Dial Transplant 25:2089–2098
Parker A, Bowles K, Bradley JA et al (2010) Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients—BCSH and BTS Guidelines. Br J Haematol 149:675–692
Tsao L, Hsi ED (2007) The clinicopathologic spectrum of posttransplantation lymphoproliferative disorders. Arch Pathol Lab Med 131:1209–1218
Taylor AL, Marcus R, Bradley JA (2005) Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol 56:155–167
Hoshida Y, Li T, Dong Z et al (2001) Lymphoproliferative disorders in renal transplant patients in Japan. Int J Cancer 91:869–875
Guthery SL, Heubi JE, Bucuvalas JE et al (2003) Determination of risk factors for Epstein-Barr virus-associated posttransplant lymphoproliferative disorder in pediatric liver transplant recipients using objective case ascertainment. Transplantation 75:987–993
Opelz G, Dohler B (2004) Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant 4:222–230
Bakker NA, van Imhoff GW, Verschuuren EA et al (2007) Presentation and early detection of post-transplant lymphoproliferative disorder after solid organ transplantation. Transpl Int 20:207–218
Shahinian VB, Muirhead N, Jevnikar AM et al (2003) Epstein-Barr virus seronegativity is a risk factor for late-onset posttranslant lymphoproliferative disorder in adult renal allograft recipients. Transplantation 75:851–856
Bakker NA, van Imhoff GW, Verschuuren EA et al (2005) HLA antigens and post renal transplant lymphoproliferative disease: HLA-B matching is critical. Transplantation 80:595–599
Walker RC, Paya CV, Marshall WF et al (1995) Pretransplantation seronegative Epstein-Barr virus státusz is the primary risk factor for posttransplantation lymphoproliferative disorder in adult heart, lung, and other solid organ transplantations. J Heart Lung Transplant 14:214–221
Capello D, Rossi D, Gaidano G (2005) Post-transplant lymphoproliferative disorders: molecular basis of disease histogenesis and pathogenesis. Hematol Oncol 23:61–67
Capello D, Rossi D, Gaudino G et al (2003) Simian virus 40 infection in lymphoproliferative disorders. Lancet 36:88–89
Cohen AH, Sweet SC, Mendeloff E et al (2000) High incidence of posttransplant lymphoproliferative disease in pediatric patients with cystic fibrosis. Am J Respir Crit Care Med 161:1252–1255
Newell KA, Alonso EM, Kelly SM et al (1997) Association between liver transplantation for Langerhans cell histiocytosis, rejection, and development of posttransplant lymphoproliferative disease in children. J Pediatr 131:98–104
Nalesnik MA, Zeevi A, Randhawa PS et al (1999) Cytokine mRNA profiles in Epstein-Barr virus-associated post-transplant lymphoproliferative disorders. Clin Transplant 13:39–44
Rossi D, Gaidano G, Gloghini A et al (2003) Frequent aberrant promoter hypermethylation of O6-methylguanine-DNA methyltransferase and death-associated protein kinase genes in immunodeficiency-related lymphomas. Br J Haematol 123:475–478
Cesarman E, Chadburn A, Liu YF et al (1998) BCL-6 gene mutations in posttransplantation lymphoproliferative disorders predict response to therapy and clinical outcome. Blood 92:2294–2302
Djokic M, Le Beau MM, Swinnen LJ et al (2006) Post-transplant lymphoproliferative disorder subtypes correlate with different recurring chromosomal abnormalities. Genes Chromosom Cancer 45:313–318
Boubenider S, Hiesse C, Groupy C et al (1997) Incidence and consequences of post-transplantation lymphoproliferative disorders. J Nephrol 10:136–145
Opelz G, Henderson R (1993) Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet 342(8886–8887):1514–1516
Younes BS, McDiarmid SV, Martin MG et al (2000) The effect of immunosuppression on posttransplant lymphoproliferative disease in pediatric liver transplant patients. Transplantation 70:94–99
Cao S, Cox KL, Berquist W et al (1999) Long-term outcomes in pediatric liver recipients: comparison between cyclospirin A and tacrolimus. Pediatr Transplant 3:22–26
Cockfield SM, Preiksaitis J, Harvey E et al (1991) Is sequential use of ALG and OKT3 in renal transplants associated with an increased incidence of fulminant posttransplant lymphoproliferative disorder? Transplant Proc 23:1106–1107
Caillard S, Dharnidharka V, Agodoa L et al (2005) Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation 80:1233–1243
Kirk AD, Cherikh WS, Ring M et al (2007) Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant 7:2619–2625
Kahan BD, Yakupoglu YK, Schoenberg L et al (2005) Low incidence of malignancy among sirolimus/cyclosporine-treated renal transplant recipients. Transplantation 80:749–758
Kahan BD, Knight R, Schoenberg L et al (2003) Ten years of sirolimus therapy for human renal transplantation: the University of Texas at Houston experience. Transplant Proc 35:25S–34S
Yakupoglu YK, Buell JF, Woodle S et al (2006) Individualization of immunosuppressive therapy. III. Sirolimus associated with a reduced incidence of malignancy. Transplant Proc 38:358–361
DiNardo CD, Tsai DE (2010) Treatment advances in posttransplant lymphoproliferative disease. Curr Opin Hematol 17:368–374
O’Neill JO, Edwards LB, Taylor DO (2006) Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 25:1186–1191
Robson R, Cecka JM, Opelz G et al (2005) Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant 5:2954–2960
Funch DP, Ko HH, Travasso J et al (2005) Posttransplant lymphoproliferative disorder among renal transplant patients in relation to the use of mycophenolate mofetil. Transplantation 80:1174–1180
Durrbach A, Pestana JM, Pearson T et al (2010) A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant 10:547–557
Pascual J (2007) Post-transplant lymphoproliferative disorder—the potential of proliferation signal inhibitors. Nephrol Dial Transplant 22:27–35
Dolcetti R (2007) B lymphocytes and Epstein-Barr virus: the lesson of post-transplant lymphoproliferative disorders. Autoimmun Rev 7:96–101
Carbone A, Gloghini A, Dotti G (2008) EBV-associated lymphoproliferative disorders: classification and treatment. Oncologist 13:577–585
Snow AL, Martinez OM (2007) Epstein-Barr virus: evasive maneuvers in the development of PTLD. Am J Transplant 7:271–277
Weiss LM, Movahed LA, Warnke RA et al (1989) Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med 320:502–506
Raab-Traub N (2002) Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol 12:431–441
Dolcetti R, Masucci MG (2003) Epstein-Barr virus: induction and control of cell transformation. J Cell Physiol 196:207–218
Pattle SB, Farrel PJ (2006) The role of Epstein-Barr virus in cancer. Expert Opin Biol Ther 6:1193–1205
Capello D, Cerri M, Muti G et al (2003) Molecular histogenesis of posttransplantation lymphoproliferative disorders. Blood 102:3775–3785
Davis JE, Sherritt MA, Bharadwaj M et al (2004) Determining virological, serological and immunological parameters of EBV infection in the development of PTLD. Int Immunol 16:983–989
Nelson BP, Nalesnik MA, Bahler DW et al (2000) Epstein-Barr virus-negative posttransplant lymphoproliferative disorders: a distinct entity ? Am J Surg Pathol 24:375–385
Penn I, Porat G (1995) Central nervous system lymphomas in organ allograft recipients. Transplantation 59:240–244
Bakker NA, van Imhoff GW, Verschuuren EA et al (2005) Early onset post-transplant lymphoproliferative disease is associated with allograft localization. Clin Transplant 19:327–334
Paranjothi S, Yusen RD, Kraus MD et al (2001) Lymphoproliferative disease after lung transplantation: comparison of presentation and outcome of early and late cases. J Heart Lung Transplant 20:1054–1063
Savoie A, Perpete C, Carpentier L et al (1994) Direct correlation between the load of Epstein-Barr virus-infected lymphocytes in the peripheral blood of pediatric transplant patients and risk of lymphoproliferative disease. Blood 83:2715–2722
Riddler SA, Breinig MC, McKnight JL (1994) Increased levels of circulating Epstein-Barr virus (EBV)-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of posttransplant lymphoproliferative disease in solid-organ transplant recipients. Blood 84:972–984
Humar A, Michaels M (2006) AST ID Working Group on Infectious Disease Monitoring. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 6:262–274
Patel S, Zuckerman M, Smith M (2003) Real-time quantitative PCR of Epstein-Barr virus BZLF1 DNA using the LightCycler. J Virol Meth 109:227–233
Fafi-Kremer S, Brengel-Pesce K, Bargues G et al (2004) Assessment of automated DNA extraction coupled with real-time PCR for measuring Epstein-Barr virus load in whole blood, peripheral mononuclear cells and plasma. J Clin Virol 30:157–164
Wagner HJ, Wessel M, Jabs W et al (2001) Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation 72:1012–1019
Tsai DE, Douglas L, Andreadis C et al (2008) EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant 8:1016–1024
Jang JY, Kim KM, Lee YJ et al (2008) Quantitative Epstein- Barr virus viral load monitoring in pediatric liver transplantation. Transplant Proc 40:2546–2548
Omar H, Hägglund H, Gustafsson-Jernberg Å et al (2009) Target monitoring of patients at high risk of post-transplant lymphoproliferative disease by quantitative Epstein-Barr virus polymerase chain reaction. Transpl Infect Dis 11:393–399
Smets F, Latinne D, Bazin H et al (2002) Ratio between Epstein-Barr viral load and anti-Epstein-Barr virus specific T-cell response as a predictive marker of posttransplant lymphoproliferative disease. Transplantation 73:1603–1610
Bianchi E, Pascual M, Nicod M et al (2008) Clinical usefulness of FDG-PET/CT scan imaging in the management of posttransplant lymphoproliferative disease. Transplantation 85:707–712
Parker A, Bowles K, Bradley JA et al (2010) Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients—BCSH and BTS Guidelines. Br J Haematol 149:693–705
Paya CV, Fung JJ, Nalesnik MA et al (1999) Epstein-Barr virus-induced posttransplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD Task Force and The Mayo Clinic Organized International Consensus Development Meeting. Transplantation 68:1517–1525
Green M, Michaels MG, Webber SA et al (1999) The management of Epstein-Barr virus associated post-transplant lymphoproliferative disorders in pediatric solid-organ transplant recipients. Pediatr Transplant 3:271–281
Gottschalk S, Rooney CM, Heslop HE (2005) Post-transplant lymphoproliferative disorders. Annu Rev Med 56:29–44
Muti G, Cantoni S, Oreste P et al (2002) Cooperative Study Group on PTLDs. Post-transplant lymphoproliferative disorders: improved outcome after clinico-pathologically tailored treatment. Haematologica 87:67–77
Elstrom RL, Andreadis C, Aqui NA et al (2006) Treatment of PTLD with rituximab or chemotherapy. Am J Transplant 6:569–576
Gross TG, Bucuvalas JC, Park JR et al (2005) Low-dose chemotherapy for Epstein-Barr virus-positive post-transplantation lymphoproliferative disease in children after solid organ transplantation. J Clin Oncol 23:6481–6488
Ohta H, Fukushima N, Ozono K (2009) Pediatric post-transplant lymphoproliferative disorder after cardiac transplantation. Int J Hematol 90:127–136
Milpied N, Vasseur B, Parquet N et al (2000) Humanized anti-CD20 monoclonal antibody (Rituximab) in post transplant B-lymphoproliferative disorder: a retrospective analysis on 32 patients. Ann Oncol 11:113–116
Ganne V, Siddiqi N, Kamaplath B et al (2003) Humanized anti-CD20 monoclonal antibody (Rituximab) treatment for post-transplant lymphoproliferative disorder. Clin Transplant 17:417–422
Trappe R, Hinrichs C, Appel U et al (2009) Treatment of PTLD with rituximab and CHOP reduces the risk of renal graft impairment after reduction of immunosuppression. Am J Transplant 9:2331–2337
Ganne V, Siddiqi N, Kamplath B et al (2003) Humanized anti-CD20 monoclonal antibody (Rituximab) treatment for post-transplant lymphoproliferative disorder. Clin Transplant 17:417–422
Heslop HE, Savoldo B, Rooney CM (2004) Cellular therapy of Epstein-Barr-virus-associated post-transplant lymphoproliferative disease. Best Pract Res Clin Haematol 17:401–413
Savoldo B, Rooney CM, Quiros-Tejeira RE et al (2005) Cellular immunity to Epstein-Barr virus in liver transplant recipients treated with rituximab for post-transplant lymphoproliferative disease. Am J Transplant 5:566–572
Kinoshita T, Nagai H, Murate T et al (1998) CD-20-negative relapse in B-cell lymphoma after treatment with Rituximab. J Clin Oncol 16:3916
Choquet S, Leblond V, Herbrecht R et al (2006) Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood 107:3053–3057
Rooney CM, Smith CA, Ng CY et al (1995) Use of gene-modified virus specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet 345:9–13
Rooney CM, Smith CA, Ng CY et al (1998) Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 92:1549–1555
Feng S, Buell JF, Chari RS et al (2003) Tumors and transplantation: the 2003 third annual ASTS state-of-the-art winter symposium. Am J Transplant 3:1481–1487
Haque T, Wilkie GM, Taylor C et al (2002) Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet 360(9331):436–442
Haque T, Wilkie GM, Jones MM et al (2007) Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 110:1123–1131
Zhan X, Brown B, Slobod KS et al (2003) Inhibition of ex vivo-expanded cytotoxic T-lymphocyte function by high-dose cyclosporine. Transplantation 76:739–740
Brewin J, Mancao C, Straathof K et al (2009) Generation of EBV-specific cytotoxic T-cells that are resistant to calcineurin inhibitors for the treatment of post-transplant lymphoproliferative disease. Blood 114:4792–4803
Davis CL, Wood BL, Sabath DE et al (1998) Interferon-alpha treatment of posttransplant lymphoproliferative disorder in recipients of solid organ transplants. Transplantation 66:1770–1779
Davis JE, Moss DJ (2004) Treatment options for post-transplant lymphoproliferative disorder and other Epstein-Barr virus-associated malignancies. Tissue Antigens 63:285–292
Haddad E, Paczesny S, Leblond V et al (2001) Treatment of B-lymphoproliferative disorder with a monoclonal anti-interleukin-6 antibody in 12 patients: a multicenter phase 1–2 clinical trial. Blood 97:1590–1597
Perrine SP, Hermine O, Small T et al (2007) A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood 109:2571–2578
Preiksaitis JK (2004) New developments in the diagnosis and management of posttransplantation lymphoproliferative disorders in solid organ transplant recipients. Clin Infect Dis 39:1016–1023
Funch DP, Walker AM, Schneider G et al (2005) Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant 5:2894–2900
Malouf MA, Chhajed PN, Hopkins P et al (2002) Anti-viral profilaxis reduces the incidence of lymphoproliferative disease in lung transplant recipients. J Heart Lung Transplant 21:547–554
Opelz G, Daniel V, Naujokat C et al (2007) Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma:a multicentre retrospective analysis. Lancet Oncol 8:212–218
Gu SY, Huang TM, Ruan L et al (1995) First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev Biol Stand 84:171–177
Posfay-Barbe KM, Siegrist CA (2009) Immunization and transplantation—what is new and what is coming? Pediatr Transplant 13:404–410
Nepomuceno RR, Balatoni CE, Natkunam Y et al (2003) Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res 63:4472–4480
Alexandru S, Gonzalez E, Grande C et al (2009) Monotherapy rapamycin in renal transplant recipients with lymphoma successfully treated with rituximab. Transplant Proc 41:2435–2437
Holmes MV, Caplin B, Atkinson C et al (2009) Prospective monitoring of Epstein-Barr virus DNA in adult renal transplant recipients during the early posttransplant period: role of mycophenolate mofetil. Transplantation 87:852–856
Vegso G, Sebestyen A, Paku S et al (2007) Antiproliferative and apoptotic effects of mycophenolic acid in human B-cell non-Hodgkin lymphomas. Leuk Res 31:1003–1008
Takebe N, Cheng X, Fandy TE et al (2006) IMP dehydrogenase inhibitor mycophenolate mofetil induces caspase-dependent apoptosis and cell cycle inhibition in multiple myeloma cells. Mol Cancer Ther 5:457–466
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Végső, G., Hajdu, M. & Sebestyén, A. Lymphoproliferative Disorders After Solid Organ Transplantation—Classification, Incidence, Risk Factors, Early Detection and Treatment Options. Pathol. Oncol. Res. 17, 443–454 (2011). https://doi.org/10.1007/s12253-010-9329-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-010-9329-8