Abstract
Neuroendocrine cells are often disclosed in human gastric adenocarcinomas and may be recognised by their immunoreactivity towards chromogranin A. However, in dedifferentiated neuroendocrine tumour cells, the chromogranin A content may be reduced making it difficult to detect with conventional immunohistochemical methods. We therefore used a sensitive signal amplification technique in order to evaluate chromogranin A immunoreactivity and thus neuroendocrine differentiation in 40 gastric adenocarcinomas.
Neuroendocrine cells were visualised by means of a monoclonal chromogranin A antibody and the avidin–biotin peroxidase complex technique, without and with addition of tyramide signal amplification. Double immunohistochemistry towards chromogranin A and Ki-67 were used to disclose proliferation in the neoplastic cells.
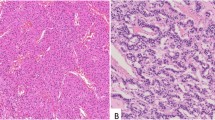
A marked increase in the number of carcinomas containing chromogranin A-immunoreactive neoplastic cells was noted when applying the tyramide signal amplification technique. In addition, the number of immunoreactive cells within each tumour increased, and in some cases almost all the neoplastic cells became immunoreactive. Chromogranin A-immunoreactive tumour cells showing signs of proliferation were found in the majority of these carcinomas.
In conclusion, we have disclosed widespread immunoreactivity towards chromogranin A in a proportion of gastric adenocarcinomas when enhancing the signal with tyramide signal amplification. Neuroendocrine differentiation is thus a common finding in gastric carcinomas when using sensitive methods.
Similar content being viewed by others
References
Adams JC (1992) Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 40: 1457–1463.
Balaton AJ, Galet BA (1998) Detection of chromogranin A mRNA in small cell lung carcinoma using a new, highly sensitive in situ hybridization method with a non-radioisotope oligonucleotide probe. Cancer 83: 1469–1470.
Bensch KG, Corrin B, Pariente R, Spencer H (1968) Oat-cell carcinoma of the lung. Its origin and relationship to bronchial carcinoid. Cancer 22: 1163–1172.
Berner A, Nesland JM (1991) Endocrine profile in gastric carcinomas. An immunohistochemical study. Histol Histopathol 6(3): 317–323.
Burry RW (2000) Specificity controls for immunocytochemical methods. J Histochem Cytochem 48(2): 163–166.
Creutzfeldt W, Arnold R, Creutzfeldt C, Deuticke U, Frerichs H, Track NS (1973) Biochemical and morphological investigations of 30 human insulinomas. Correlation between the tumour content of insulin and proinsulin-like components and the histological and ultrastructural appearance. Diabetologia 9: 217–231.
Deftos LJ, Linnoila RI, Carney DN, Burton DW, Leong SS, O'Connor DT, Murray SS, Gazdar AF (1988) Demonstration of Chromogranin A in human neuroendocrine cell lines by immunohistology and immunoassay. Cancer 62: 92–97.
Gould VE, Linnoila RI, Memoli VA, Warren WH (1983) Neuroendocrine cells and neuroendocrine neoplasms of the lung. Pathol Annu 18: 287–330.
Laurén P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand 64: 31–49.
Lengauer C, Kinzler KW, Vogelstein B (1998) Genetic instabilities in human cancers. Nature 396: 643–649.
Linnoila RI, Mulshine JL, Steinberg SM, Funa K, Matthews MJ, Cotelingam JD, Gazdar AF (1988) Neuroendocrine differentiation in endocrine and nonendocrine lung carcinomas. Am J Clin Pathol 90: 641–652.
Merz H, Malisius R, Mannweiler S, Zhou R, Hartmann W, Orscheschek K, Moubayed P, Feller AC (1995) ImmunoMax. A maximized immunohistochemical method for the retrieval and enhancement of hidden antigens. Lab Invest 73: 149–156.
Nevalainen TJ, Laurén PA (1984) Endocrine cells in gastric carcinoma. Tumor Res 19: 21–24.
Ooi A, Hayashi H, Katsuda S, Nakanishi I (1992) Gastric carcinoma cells with endocrine differentiation show no evidence of proliferation. Hum Pathol 23: 736–741.
Ooi A, Mai M, Ogino T, Ueda H, Kitamura T, Takahashi Y, Kawahara E, Nakanishi I (1988) Endocrine differentiation of gastric adenocarcinoma. The prevalence as evaluated by immunoreactive chromogranin A and its biologic significance. Cancer 62: 1096–1104.
Qvigstad G, Falkmer S, Westre B, Waldum HL (1999) Clinical and histopathological tumour progression in ECL cell carcinoids ('ECLomas'). APMIS 107: 1085–1093.
Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E (1993) Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 104: 994–1006.
Sumiyoshi Y, Shirakusa T, Yamashita Y, Maekawa T, Hideshima T, Sakai T, Kawahara K, Kikuchi M (1998) Detection of chromogranin A mRNAin small cell lung carcinoma using a new, highly sensitive in situ hybridization method with a non-radioisotope oligonucleotide probe. Cancer 82: 468–473.
Waldum HL, Haugen OA, Isaksen C, Mescei R, Sandvik AK (1991) Are diffuse gastric carcinomas neuroendocrine tumours (ECL-omas)? Eur J Gastroenterol Hepatol 3: 245–249.
Waldum HL, Rørvik H, Brenna E (1996) The gastrointestinal tract and the lungs. Similarities with particular emphasis on the neuroendocrine cells. Sarcoidosis Vasc Diffuse Lung Dis 13(1): 63–65.
Waldum HL, Aase S, Kvetnoi I, Brenna E, Sandvik AK, Syversen U, Johnsen G, Vatten L, Polak JM (1998) Neuroendocrine differentiation in human gastric carcinomas. Cancer 83(3): 435–444.
Whitehead R, Cosgrove C (1979) Mucins and carcinoid tumours. Pathology 11: 473–478.
Wiedorn KH, Kühl H, Galle J, Caselitz J, Vollmer E (1999) Comparison of in situ hybridization, direct and indirect in situ PCR as well as tyramide signal amplification for the detection of HPV. Histochem Cell Biol 111(2): 89–95.
Wilson BS, Lloyd RV (1984) Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol 115: 458–468.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Qvigstad, G., Sandvik, A.K., Brenna, E. et al. Detection of Chromogranin A in Human Gastric Adenocarcinomas using a Sensitive Immunohistochemical Technique. Histochem J 32, 551–556 (2000). https://doi.org/10.1023/A:1004102312006
Issue Date:
DOI: https://doi.org/10.1023/A:1004102312006