Abstract
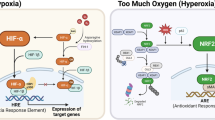
As a result of deprivation of oxygen (hypoxia) and nutrients, the growth and viability of cells is reduced1. Hypoxia-inducible factor(HIF)-1α helps to restore oxygen homeostasis by inducing glycolysis, erythropoiesis and angiogenesis2,3,4. Here we show that hypoxia and hypoglycaemia reduce proliferation and increase apoptosis in wild-type (HIF-1α+/+) embryonic stem (ES) cells, but not in ES cells with inactivated HIF-1α genes (HIF-1α−/−); however, a deficiency of HIF-1α does not affect apoptosis induced by cytokines. We find that hypoxia/hypoglycaemia-regulated genes involved in controlling the cell cycle are either HIF-1α-dependent (those encoding the proteins p53, p21, Bcl-2) or HIF-1α-independent (p27, GADD153), suggesting that there are at least two different adaptive responses to being deprived of oxygen and nutrients. Loss of HIF-1α reduces hypoxia-induced expression of vascular endothelial growth factor, prevents formation of large vessels in ES-derived tumours, and impairs vascular function, resulting in hypoxic microenvironments within the tumour mass. However, growth of HIF-1α tumours was not retarded but was accelerated, owing to decreased hypoxia-induced apoptosis and increased stress-induced proliferation. As hypoxic stress contributes to many (patho)biological disorders1,5, this new role for HIF-1α in hypoxic control of cell growth and death may be of general pathophysiological importance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bunn, H. F. & Poyton, R. O. Oxygen sensing and molecular adaptation to hyopoxia. Physiol. Rev. 76, 839–885 (1996).
Wenger, R. H. & Gassmann, M. Oxygen(s) and the hypoxia-inducible factor-1. Biol. Chem. 378, 609–616 (1997).
Semenza, G. L. Transcriptional regulation by hypoxia-inducible factor-1. Trends Cardiovasc. Med. 6, 151–157 (1996).
Iyer, N. V.et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor-1α. Genes Dev. 12, 149–162 (1998).
Dor, Y. & Keshet, E. Ischemia-driven angiogenesis. Trends Cardiovasc. Med. 7, 289–294 (1997).
Gartel, A. L., Serfas, M. S. & Tyner, A. L. p21–negative regulator of the cell cycle. Proc. Soc. Exp. Biol. Med. 213, 138–149 (1996).
Toyoshima, H. & Hunter, T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell 78, 67–74 (1994).
Price, B. D. & Calderwood, S. K. Gadd45 and Gadd153 messenger RNA levels are increased during hypoxia and after exposure of cells to agents which elevate the levels of the glucose-regulated proteins. Cancer Res. 52, 3814–3817 (1992).
Little, E., Ramakrishnan, M., Roy, B., Gazit, G. & Lee, A. S. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryot. Gene Expr. 4, 1–18 (1994).
Wood, S. M.et al. Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1α subunit. Characterization of HIF-1α dependent and independent hypoxia-inducible gene expression. J. Biol. Chem. 273, 8360–8367 (1998).
Liebermann, D. A., Hoffman, B. & Steinman, R. A. Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene 11, 199–210 (1995).
Cox, L. S. Multiple pathways control cell growth and transformation: overlapping and independent activities of p53 and p21Cip1/WAF1/Sdi1. J. Pathol. 183, 134–140 (1997).
Graeber, T. G.et al. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol. Cell. Biol. 14, 6264–6277 (1994).
Strasser, A., Huang, D. C. & Vaux, D. L. The role of the bcl-2/ced-9 gene family in cancer and general implications of defects in cell death control for tumorigenesis and resistance to chemotherapy. Biochim. Biophys. Acta 1333, F151–178 (1997).
Maxwell, P. H.et al. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc. Natl Acad. Sci. USA 94, 8104–8109 (1997).
Maltepe, E., Schmidt, J. V., Baunoch, D., Bradfield, C. A. & Simon, C. M. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386, 403–407 (1997).
Schmaltz, C., Harrigan Hardenbergh, P., Wells, A. & Fisher, D. E. Regulation of proliferation–survival decisions during tumor cell hypoxia. Mol. Cell. Biol. 18, 2845–2854 (1998).
Collins, M. K., Perkins, G. R., Rodriguez Tarduchy, G., Nieto, M. A. & Lopez Rivas, A. Growth factors as survival factors: regulation of apoptosis. Bioessays 16, 133–138 (1994).
Lin, Y. & Benchimol, S. Cytokines inhibit p53-mediated apoptosis but not p53-mediated G1 arrest. Mol. Cell. Biol. 15, 6045–6054 (1995).
Vaupel, P. The influence of tumor blood flow and microenvironmental factors on the efficacy of radiation, drugs and localized hyperthermia. Klin. Pädiatr. 209, 243–249 (1997).
Yuan, F.et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc. Natl Acad. Sci. USA 93, 14765–14770 (1996).
Graeber, T. G.et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379, 88–91 (1996).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and p O2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Med. 3, 177–182 (1997).
An, W. G.et al. Stabilization of wild-type p53 by hypoxia-inducible factor-1α. Nature 392, 405–408 (1998).
Jiang, B. H., Rue, E., Wang, G. L., Roe, R. & Semenza, G. L. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor-1. J. Biol. Chem. 271, 17771–17778 (1996).
Carmeliet, P.et al. Abnormal blood vessel development and lethality in embryos lacking a single vascular endothelial growth-factor allele. Nature 380, 435–439 (1996).
Abramovitch, R., Frenkiel, D. & Neeman, M. Analysis of subcutaneous angiogenesis by gradient echo magnetic resonance imaging. Magn. Reson. Med. 39, 813–824 (1998).
Kobayashi, N., Kobayashi, K., Kouno, K., Horinaka, S. & Yagi, S. Effects of intra-arterial injection of colored microspheres on systemic hemodynamics and regional blood flow in rats. Am. J. Physiol. 266, H1910–H1917 (1994).
Evans, S. M.et al. Identification of hypoxia in cells and tissues of epigastric 9L rat glioma using EF5 (2-(2-nitro-1H-imidazol-1-yl)- N -(2,2,3,3,3-pentafluoropropyl) acetamide). Br. J. Cancer 72, 875–882 (1995).
Herbert, J. M. & Carmeliet, P. Involvement of u-PA in the anti-apoptotic activity of TGFβ for vascular smooth muscle cells. FEBS Lett. 413, 401–404 (1997).
Acknowledgements
We thank D. Livingston for monoclonal HIF-1α antibodies; M. Lampugnani and E.Dejana for CD31 antibodies; E. M. Lord for ALK3.51 antibodies; G. Suske for Sp1 antibodies; S.Plaisance and G. Theilmeier for discussion and for their help; K. Bijnens, A. Bouché, I. Cornelissen, M.De Mol, E. Gils, B. Hermans, S. Jansen, L. Kieckens, A. Manderveld, T. Vancoetsem, A. Vandenhoeck, A. Van den Boomen, P. Van Wesemael, S. Wyns (Leuven), A. Itin, I. Lamarche, P. Rogon, Y. Chen, J. Kahn and T.Gohongi for technical assistance; and M. Deprez for artwork. This work was supported in part by a NCI-OIG grant to R.J. and D.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carmeliet, P., Dor, Y., Herbert, JM. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998). https://doi.org/10.1038/28867
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/28867
This article is cited by
-
METTL3 facilitates renal cell carcinoma progression by PLOD2 m6A-methylation under prolonged hypoxia
Cell Death & Disease (2024)
-
LncRNA SATB2-AS1 overexpression represses the development of hepatocellular carcinoma through regulating the miR-3678-3p/GRIM-19 axis
Cancer Cell International (2023)
-
Targeting hypoxia-inducible factors: therapeutic opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Design and cytotoxic evaluation via apoptotic and antiproliferative activity for novel 11(4-aminophenylamino)neocryptolepine on hepatocellular and colorectal cancer cells
Apoptosis (2023)
-
Could senescence phenotypes strike the balance to promote tumor dormancy?
Cancer and Metastasis Reviews (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.