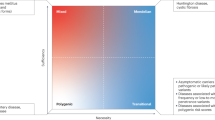
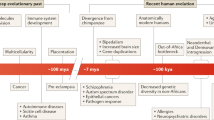
Key Points
-
The inherited disorders of haemoglobin are the commonest genetic diseases.
-
These diseases consist of the structural haemoglobin variants, such as sickle-cell haemoglobin, and the thalassaemias, which result in defective globin production.
-
The β-thalassaemias are causing an increasingly important public health problem throughout tropical countries.
-
The severity and clinical symptoms of the β-thalassaemias are extremely variable.
-
Much of the clinical variability of the β-thalassaemias can be accounted for by the complex interactions of the primary thalassaemia mutations, with various secondary and tertiary genetic modifiers and with environmental factors.
-
A better understanding of the mechanisms that underlie the phenotypic diversity of this disease offers hope for improved genetic counselling and ways towards new treatment strategies.
-
Furthering our understanding of the clinically diverse monogenic disorders should help inform future experimental approaches to unravelling the genetics and pathophysiology of the more common complex diseases.
Abstract
The remarkable phenotypic diversity of the β-thalassaemias reflects the heterogeneity of mutations at the β-globin locus, the action of many secondary and tertiary modifiers, and a wide range of environmental factors. It is likely that phenotype–genotype relationships will be equally complex in the case of many monogenic diseases. These findings highlight the problems that might be encountered in defining the relationship between the genome and the environment in multifactorial disorders, in which the degree of heritability might be relatively low and several environmental agents are involved. They also emphasize the value of an understanding of phenotype–genotype relationships in designing approaches to gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weatherall, D. J. & Clegg, J. B. The Thalassaemia Syndromes 4th edn (Blackwell Science, Oxford, 2001).The principal monograph on thalassaemia: the fourth edition covers every aspect of the field and contains over 3,000 references.
Weatherall, D. J. & Clegg, J. B. Thalassaemia — a global public health problem. Nature Med. 2, 847–849 (1996).
Weatherall, D. J., Clegg, J. B., Higgs, D. R. & Wood, W. G. in The Metabolic and Molecular Bases of Inherited Disease (ed. Scriver, C. R.) 4571–4636 (McGraw–Hill, New York, 2001).
Stamatoyannopoulos, G. & Grosveld, F. in The Molecular Basis of Blood Disease (eds Stamatoyannopoulos, G., Majerus, P. W., Perimutter, R. M. & Varmus, H.) 135–182 (Saunders, New York, 2001).An excellent up-to-date review on what is known about the mechanisms of the differential expression of the globin genes during development.
World Health Organization. Guidelines for the control of haemoglobin disorders. Report of the VIth Annual Meeting of the WHO Working Group on Haemoglobinopathies, Cagliari, Sardinia, 8–9 April, 1989 (1994).
Huisman, T. H. J., Carver, M. F. H. & Efremov, G. D. A Syllabus of Human Hemoglobin Variants (Sickle Cell Foundation, Augusta, Georgia, 1998).
Higgs, D. R. in Baillière's Clinical Haematology. International Practice and Research: The Haemoglobinopathies (eds Higgs, D. R. & Weatherall, D. J.) 117–150 (Baillière Tindall, London, 1993).
Orkin, S. H. et al. Abnormal RNA processing due to the exon mutation of βE-globin gene. Nature 300, 768– 769 (1982).
Weatherall, D. J. Pathophysiology of thalassaemia. Clin. Haematol. 11 , 127–146 (1998).
Weatherall, D. J. et al. A genetically determined disorder with features both of thalassaemia and congenital dyserythropoietic anaemia. Br. J. Haematol. 24, 681–702 (1973).
Stamatoyannopoulos, G., Woodson, R., Papayannopoulou, T., Heywood, D. & Kurachi, M. S. Inclusion-body β-thalassemia trait. A form of β thalassemia producing clinical manifestations in simple heterozygotes. N. Engl. J. Med. 290, 939 –943 (1974).
Wainscoat, J. S., Thein, S. L. & Weatherall, D. J. Thalassaemia intermedia. Blood Rev. 1, 273–279 (1987).
Fiorelli, G., Sampietro, M., Romano, M., Albano, M. & Cappellini, M. D. Clinical features of thalassemia intermedia in Italy. Birth Defects: Original Articles Ser. 23, 287–295 (1988).
Cao, A., Gasperini, D., Podda, A. & Galanello, R. Molecular pathology of thalassemia intermedia. Eur. J. Int. Med. 1, 227–236 (1990).
Rund, D. et al. Genetic analysis of β-thalassemia intermedia in Israel: diversity of mechanisms and unpredictability of phenotype. Am. J. Hematol. 54, 16–22 ( 1997).
Ho, P. J., Hall, G. W., Luo, L. Y., Weatherall, D. J. & Thein, S. L. β thalassaemia intermedia: is it possible to predict phenotype from genotype? Br. J. Haematol. 100 , 70–78 (1998). The most complete analysis so far of the molecular mechanisms for the milder forms of β-thalassaemia.
Huisman, T. H. J., Carver, M. F. H. & Baysal, E. A Syllabus of Thalassemia Mutations 1– 309 (The Sickle Cell Anemia Foundation, Augusta, Georgia, 1997).A complete and detailed catalogue of all the published thalassaemia mutations and their geographical distribution up to 1997.
Schwartz, E. The silent carrier of β thalassemia. N. Engl. J. Med. 281, 1327–1333 (1969).
Gonzalez-Redondo, J. H. et al. A CØT substitution at nt-101 in a conserved DNA sequence of the promoter region of the β-globin gene is associated with 'silent' β-thalassemia . Blood 73, 1705–1711 (1989).
Bianco, I. et al. Silent thalassemias: genotypes and phenotypes. Haematologica 82, 269–280 (1997).
Gonzalez-Redondo, J. H. et al. Clinical and genetic heterogeneity in black patients with homozygous β-thalassemia from the Southeastern United States. Blood 72, 1007–1014 ( 1988).
Tamagnini, G. P., Lopes, M. C., Castanheira, M. E., Wainscoat, J. S. & Wood, W. G. β+ thalassaemia — Portuguese type: clinical, haematological and molecular studies of a newly defined form of β thalassaemia. Br. J. Haematol. 54, 189–200 (1983).
Efremov, D. et al. Possible factors influencing the haemoglobin and fetal haemoglobin levels in patients with β-thalassaemia due to a homozygosity for the IVS-1-6 (T-C) mutation. Br. J. Haematol. 86, 824–830 (1994).
Thein, S. L. et al. Molecular basis for dominantly inherited inclusion body β thalassemia. Proc. Natl Acad. Sci. USA 87, 3924–3928 (1990).
Ho, P. J. et al. Erythroblastic inclusions in dominantly inherited β thalassaemias . Blood 89, 322–328 (1997).
Kazazian, H. H., Dowling, C. E., Hurwitz, R. L., Coleman, M. & Adams, J. G. I. Thalassemia mutations in exon 3 of the β-globin gene often cause a dominant form of thalassemia and show no predilection for malarial-endemic regions of the world. Am. J. Hum. Genet. 45, A242 ( 1989).
Thein, S. L. Dominant β thalassaemia: molecular basis and pathophysiology. Br. J. Haematol. 80, 273–277 (1992).
Thein, S. L. Is it dominantly inherited β thalassaemia or just a β-chain variant that is highly unstable? Br. J. Haematol. 107, 12–21 (1999).An extensive review of the pathophysiology of the dominant β-thalassaemias.
Sachs, A. B. Messenger RNA degradation in eukaryotes. Cell 74, 413–421 (1993).
Thermann, R. et al. Binary specification of nonsense codons by splicing and cytoplasmic translation. EMBO J. 17, 3484– 3494 (1998).
Zhang, J., Sun, X., Qian, Y. & Maquat, L. E. Intron function in the nonsense-mediated decay of β-globin mRNA: indications that pre-mRNA splicing in the nucleus can influence mRNA translation in the cytoplasm. RNA 4, 801–815 ( 1998).
Cividalli, G., Kerem, H., Execkiel, E. & Rachmilewitz, E. A. β°-thalassemia intermedia. Blood 52, 345– 349 (1978).
Weatherall, D. J. et al. The clinical and molecular heterogeneity of the thalassaemia syndromes. Ann. NY Acad. Sci. 344, 83– 100 (1980).
Rees, D. C., Styles, J., Vichinsky, E. P., Clegg, J. B. & Weatherall, D. J. The hemoglobin E syndromes . Ann. NY Acad. Sci. 850, 334– 343 (1998).
De Silva, S. et al. Thalassaemia in Sri Lanka: implications for the future health burden of Asian populations. Lancet 355, 786–791 (2000).
Kan, Y. W. & Nathan, D. G. Mild thalassemia: the result of interactions of α and β thalassemia genes. J. Clin. Invest. 49, 635–642 ( 1970).
Galanello, R. et al. Molecular analysis of β°-thalassemia intermedia in Sardinia. Blood 74, 823– 827 (1989).
Weatherall, D. J., Pressley, L., Wood, W. G., Higgs, D. R. & Clegg, J. B. Molecular basis for mild forms of homozygous beta-thalassaemia. Lancet 1 , 527–529 (1981).
Knox-Macaulay, H. H. M., Weatherall, D. J., Clegg, J. B., Bradley, J. & Brown, M. J. Clinical and biosynthetic characterization of αβ-thalassaemia. Br. J. Haematol. 22, 497–512 (1972).
Boyer, S. H., Belding, T. K., Margolet, L. & Noyes, A. N. Fetal hemoglobin restriction to a few erythrocytes (F cells) in normal human adults. Science 188, 361– 363 (1975).
Rees, D. C., Porter, J. B., Clegg, J. B. & Weatherall, D. J. Why are hemoglobin F levels increased in Hb E/β thalassemia? Blood 94, 3199–3204 ( 1999).
Garner, C. et al. Genetic influences on F cells and other hematologic variables: a twin heritability study. Blood 95, 342 –346 (2000).The most extensive study so far on the genetic factors that modify the small amounts of fetal haemaglobin that are produced in normal adults.
Gilman, J. G. & Huisman, T. H. J. DNA sequence variation associated with elevated fetal Gγ globin production. Blood 66, 783–787 ( 1985).
Labie, D. et al. Common haplotype dependency of high Gγ-globin gene expression and high Hb F levels in β-thalassemia and sickle cell anemia patients. Proc. Natl Acad. Sci. USA 82, 2111–2114 (1985).
Thein, S. L. et al. Association of thalassaemia intermedia with a beta-globin gene haplotype. Br. J. Haematol. 65, 367 –373 (1987).
Thein, S. L. in Baillière's Clinical Haematology. International Practice and Research: The Haemoglobinopathies (eds Higgs, D. R. & Weatherall, D. J.) 151–176 (Baillière Tindall, London, 1993).
Thein, S. L. & Weatherall, D. J. in Hemoglobin Switching. B. Cellular and Molecular Mechanisms (eds Stamatoyannopoulos, G. & Nienhuis, A. W.) 97–112 (Alan R. Liss, New York, 1989).
Craig, J. E. et al. Haemoglobin switch: dissecting the loci controlling fetal haemoglobin production on chromosomes 11p and 6q by the regressive approach . Nature Genet. 12, 58– 64 (1996).A model approach to dissecting the loci that modify fetal haemaglobin production in the haemoglobin disorders.
Craig, J. E. et al. Genetic heterogeneity in heterocellular hereditary persistence of fetal hemoglobin. Blood 90, 428– 434 (1997).
Dover, G. J. et al. Fetal hemoglobin levels in sickle cell disease and normal individuals are partially controlled by an X-linked gene located at Xp22.2 . Blood 80, 816–824 (1992).
Higgs, D. R., Old, J. M., Pressley, L., Clegg, J. B. & Weatherall, D. J. A novel α-globin gene arrangement in man. Nature 284, 632–635 ( 1980).
Goossens, M. et al. Triplicated α-globin loci in humans. Proc. Natl Acad. Sci. USA 77, 518–521 (1980).
Kanavakis, E., Metaxatou-Mavromati, A., Kattamis, C., Wainscoat, J. S. & Wood, W. G. The triplicated α gene locus and β thalassaemia. Br. J. Haematol. 54, 201–207 (1983).
Galanello, R. et al. A family with segregating triplicated α globin loci and β thalassemia. Blood 62, 1035– 1040 (1983).
Thompson, C. C., Ali, M. A. & Vacovsky, M. The interaction of anti 3.7 type quadruplicated α-globin genes and heterozygous β-thalassemia. Hemoglobin 13, 125–135 (1989).
Beris, P. et al. Severe inclusion body β-thalassaemia with haemolysis in a patient double heterozygous for β°-thalassaemia and quadruplicated α-globin gene arrangement of the anti-4.2 type. Br. J. Haematol. 105, 1074–1080 (1999).
Galanello, R. et al. Hyperbilirubinaemia in heterozygous β-thalassaemia is related to co-inherited Gilbert's syndrome. Br. J. Haematol. 99, 433–436 (1997).
Sampietro, M. et al. The expression of uridine diphosphate glucuronosyltransferase gene is a major determinant of bilirubin level in heterozygous β-thalassaemia and in glucose-6-phosphate dehydrogenase deficiency. Br. J. Haematol. 99, 437–439 ( 1997).
Rees, D. C., Singh, B. M., Luo, L. Y., Wickramasinghe, S. & Thein, S. L. Nontransfusional iron overload in thalassemia: association with hereditary hemochromatosis. Ann. NY Acad. Sci. 850, 490–494 (1998).
Piperno, A. et al. Haemochromatosis in patients with β-thalassaemia trait . Br. J. Haematol. 111, 908– 914 (2000).
Merryweather-Clarke, A. T., Pointon, J. J., Shearman, J. D. & Robson, K. J. H. Global prevalence of putative haemochromatosis mutations. J. Med. Genet. 34, 275–278 (1997).
Andrews, N. C. Iron homeostasis: insights from genetics and animal models. Nature Rev. Genet. 1, 208–217 ( 2000).An extensive review of new insights into iron homeostasis that discusses the many gene candidates for modifiers of iron loading.
Rees, D. C. et al. Genetic influences on bone disease in thalassemia. Blood 92, S532a (1998).
Wonke, B. Annotation: bone disease in β-thalassaemia major. Br. J. Haematol. 103, 897–901 ( 1998).An up-to-date outline of the acquired and potential genetic factors that might modify osteoporosis in β-thalassaemia.
Perrota, S. et al. Osteoporosis in β thalassaemia major patients: analysis of the genetic background. Br. J. Haematol. 111, 461–466 (2000).
Dresner Pollak, R., Rachmilewitz, E., Blumenfeld, A., Idelson, M. & Goldfarb, A. W. Bone mineral metabolism in adults with β-thalassaemia major and intermedia. Br. J. Haematol. 111, 902–907 ( 2000).
Williams, T. N. et al. High incidence of malaria in α-thalassaemic children . Nature 383, 522–525 (1996).
Allen, S. J. et al. α+-thalassaemia protects children against disease due to malaria and other infections. Proc. Natl Acad. Sci. USA 94, 14736–14741 ( 1997).
Weatherall, D. J., Clegg, J. B. & Kwiatkowski, D. The role of genomics in studying genetic susceptibility to infectious disease. Genome Res. 7, 967 –973 (1997).An extensive review of the malaria-related genetic polymorphisms and polymorphisms that alter susceptibility to other infectious agents.
Hill, A. V. S., Allsopp, C. E. M. & Kwiatkowski, D. Common west African HLA antigens are associated with protection from severe malaria. Nature 352, 595–600 (1991).
McGuire, W., Hill, A. V. S., Allsopp, C. E. M., Greenwood, B. M. & Kwiatkowski, D. Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature 371, 508– 511 (1994).
Fernandez-Reyes, D. et al. A high frequency African coding polymorphism in the N-terminal domain of ICAM-1 predisposing to cerebral malaria in Kenya. Hum. Mol. Genet. 6, 1357–1360 (1997).
Weatherall, D. J. Thalassemia in the next millennium. Ann. NY Acad. Sci. 850, 1–9 (1998).
Fouladi, M. et al. Hemoglobin E/β thalassemia: the Canadian experience. Ann. NY Acad. Sci. 850, 410–411 (1998).
Rees, D. C., Clegg, J. B. & Weatherall, D. J. Is hemoglobin instability important in the interaction between hemoglobin E and β thalassemia? Blood 92, 2141–2146 (1998).
Chinprasertsuk, S., Wanachiwanawin, W. & Piankijagum, A. Effect of pyrexia in the formation of intraerythrocytic inclusion bodies and vacuoles in haemolytic crisis of haemoglobin H disease . Eur. J. Haematol. 52, 87– 91 (1994).
Weatherall, D. J. From genotype to phenotype: genetics and medical practice in the new millennium . Phil. Trans. R. Soc. Lond. B 354, 1995 –2010 (1999).An extensive review of phenotype–genotype relationships in monogenic disease, including those other than the haemoglobin disorders.
Summers, K. M. Relationship between genotype and phenotype in monogenic diseases: relevance to polygenic diseases. Hum. Mutat. 7, 283 –293 (1996).
Wolf, U. Identical mutations and phenotypic variation. Hum. Genet. 100, 305–321 (1997). A valuable review of the phenotype–genotype relationships for monogenic disease.
Bunn, H. F. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 337, 762–769 ( 1997).
Weatherall, D. J. Gene therapy: repairing haemoglobin disorders with ribozymes. Curr. Biol. 8, R696–R698 (1998).
Acknowledgements
This work was supported by the Medical Research Council (MRC) and the Wellcome Trust. I thank my former colleagues in the MRC Molecular Haematology Unit for their help and support.
Author information
Authors and Affiliations
Related links
Related links
DATABASE LINKS
hereditary persistence of fetal haemoglobin
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- THALASSAEMIA
-
Inherited disorder caused by the abnormal production of haemoglobin.
- SPLENOMEGALY
-
Enlargement of the spleen that results in the pooling of red cells and in anaemia.
- ANAEMIA
-
A reduction in the haemoglobin level or red-cell count, which leads to defective tissue oxygenation.
- INTERCURRENT ILLNESS
-
An illness unrelated to the primary disease (for example, infection or malnutrition in a child with thalassaemia).
- OSTEOPOROSIS
-
Reduction in the amount of bone without a change in its composition. Associated with bone pain and fractures.
- HAEMOLYTIC ANAEMIA
-
Anaemia due to reduced red-cell survival.
- ERYTHROPOIESIS
-
Differentiation and maturation of red blood cells.
- BILIRUBIN
-
A principal metabolic product of haemoglobin breakdown.
- HYPOGONADISM
-
Reduction in ovarian or testicular function. This might be primary, due to disease of the ovaries or testes, or secondary due to disease of the hypothalamic–pituitary axis.
Rights and permissions
About this article
Cite this article
Weatherall, D. Phenotype—genotype relationships in monogenic disease: lessons from the thalassaemias . Nat Rev Genet 2, 245–255 (2001). https://doi.org/10.1038/35066048
Issue Date:
DOI: https://doi.org/10.1038/35066048
This article is cited by
-
Base-editing-mediated dissection of a γ-globin cis-regulatory element for the therapeutic reactivation of fetal hemoglobin expression
Nature Communications (2022)
-
Evaluation of β-Thalassaemia Cases for Common Mutations in Western Rajasthan
Indian Journal of Hematology and Blood Transfusion (2021)
-
Presentation of two new mutations in the 3′untranslated region of the β-globin gene and evaluating the molecular spectrum of thalassemia mutations in the Mediterranean region of Turkey
Annals of Hematology (2021)
-
Identifying genetic variants and pathways associated with extreme levels of fetal hemoglobin in sickle cell disease in Tanzania
BMC Medical Genetics (2020)
-
Hepcidin gene polymorphisms and iron overload in β-thalassemia major patients refractory to iron chelating therapy
BMC Medical Genetics (2020)