Abstract
Immunologic checkpoint blockade with antibodies against the programmed cell death protein-1 (PD-1) or its ligand (PD-L1) is an effective method for reversing cancer immunosuppression and thereby promoting immune responses against several cancer types. Anti-PD-1 and anti-PD-L1 antibodies have resulted in long-term responses with minimal side effects in significant numbers of patients with melanoma, lung, kidney, bladder and triple-negative breast cancer, as well as in chemotherapy-refractory Hodgkin disease. There is already evidence from at least one randomised trial that anti-PD-1 therapy is superior to chemotherapy in the treatment of patients with metastatic melanoma, and two anti-PD-1 antibodies, pembrolizumab and nivolumab, have been approved by the US Food and Drug Administration for the treatment of patients previously treated for metastatic melanoma. It is anticipated that approvals by drug regulatory bodies will be forthcoming in several cancers in the next months.
Similar content being viewed by others
Main
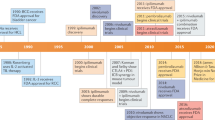
Blockade of cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 or its ligand (PD-1/L1) represent a paradigm shift in immunotherapy for cancer, as it focus on the disinhibition of native immune responses instead of the prior focus in activation of the immune system with tumour vaccines or recombinant cytokines. Among the most promising approaches to activating therapeutic antitumour immunity is the blockade of immune checkpoints. CTLA-4 was the first negative regulatory checkpoint receptor to be clinically targeted. CTLA-4 is upregulated early during the T-cell activation and its expression dampens T cells by outcompeting CD28 in binding CD80 and CD86 (Linsley et al, 1994; Egen and Allison, 2002; Riley et al, 2002). Antibodies that block CTLA-4 enhance immune responses by activating effector T cells, but probably also by interacting with other immune cells such as regulatory T cells (Tregs), which exhibit immunosuppressive properties (Lenschow et al, 1996; Wing et al, 2008). The anti-CTLA-4 antibody ipilimumab (Yervoy; Bristol-Myers Squibb, Princeton, NJ, USA) showed a significant overall survival (OS) improvement in patients with advanced melanoma in two randomised phase III trials (Hodi et al, 2010; Robert et al, 2011), and was approved by the US Food and Drug Administration (FDA) and other drug regulatory bodies in 2011. The broad activation of the immune system and deregulation of an immunologic homoeostasis achieved by blocking CTLA-4 might be responsible for the development of inflammatory or autoimmune toxicities, reported in ∼15% of the patients (Robinson et al, 2004).
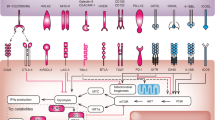
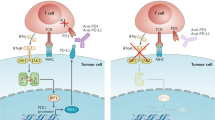
In contrast, PD-1 appears to have a prominent role in modulating T-cell activity in peripheral tissues via interaction with its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC). Programmed cell death protein-1 is an immune checkpoint receptor that prevents overstimulation of immune responses and contributes to the maintenance of immune tolerance to self-antigens (Freeman et al, 2000; Keir et al, 2006; Korman et al, 2006; Okazaki and Honjo, 2007). Upon antigen recognition, activated T cells express PD-1 on their surface and produce interferons that lead to the expression of PD-L1 in multiple tissues, including cancer (Ishida et al, 1992; Pardoll, 2012). Binding of PD-1 to its ligands limits effector T-cell activity, and therefore regulating detrimental immune responses and preventing autoimmunity (Topalian et al, 2012a). Programmed cell death protein-1 is not only induced on effector T cells but also on Tregs (Francisco et al, 2009), activated B cells and natural killer cells (Terme et al, 2011), suggesting its contribution to other important immune cell functions.
Besides the interaction between CTLA-4 and PD-1 with their respective ligands, other costimulatory and inhibitory interactions regulate T-cell responses. Although not the focus of the current review, examples of promising inhibitors of immune checkpoint targets that are being pursued clinically using blocking antibodies include the lymphocyte-activation gene 3, the T-cell membrane protein 3 or the adenosine receptor A2aR.
Regulation of expression of PD-1 and its ligands
For PD-1 to inhibit effector T-cell function, engagement to its ligands is needed. Peripheral tissues that are able to express PD-L1 constitutively or upon interferon exposure include both haematopoietic and non-haematopoietic tissues. Numerous tumour types are also able to express PD-L1 (Zou and Chen, 2008), including urothelial, ovarian, breast, cervical, colorectal, pancreatic, gastric cancer, melanoma, glioblastoma and non-small-cell lung cancer (NSCLC), suggesting that the pathway may be involved in immune evasion by many different human cancers. Less is known about PD-L2, which is expressed on dendritic cells, macrophages, mast cells and B cells (Topalian et al, 2012b). In tumours, PD-L2 is upregulated on primary mediastinal B-cell lymphoma, follicular B-cell lymphoma and Hodgkin lymphoma (Rosenwald et al, 2003).
An important consideration is the mechanism that contributes to PD-L1 expression in the surface of tumour cells. Tumour PD-L1 membrane expression can be constitutive through oncogenic processes (Parsa et al, 2007; Pardoll, 2012) or induced by activated tumour antigen-specific T cells that produce interferons (Taube et al, 2012). Thus, the expression of PD-L1 can be considered a dynamic process during effector T-cell antigen recognition.
Recent data suggest that inducible PD-L1 expression may be most important for responses to PD-1 blockade therapy. In this scenario, the pre-existing presence of PD-1-positive T cells with tumour antigen specificity, which became inactivated upon PD-L1 engagement, is required for antitumour responses. This critical mechanism has been termed acquired immune resistance (Pardoll, 2012), and differs from other possible scenarios where tumour cells express PD-L1 in the absence of effector T cells or effector T cells are present but not properly activated and therefore not able to express PD-1 (Ribas and Tumeh, 2014).
Tumour infiltration with effector T cells and PD-L1 expression have been associated with objective responses to the anti-PD1 antibody pembrolizumab in biopsies of patients with advanced melanoma (Tumeh et al, 2014). A more clonal TCR receptor repertoire in the previous patients with clinical benefit has also been observed. Other work focused on inducible PD-L1 expressed by tumour-infiltrating T cells as predictive of response, which is also likely a reflection of the presence of tumour antigen-specific T cell-producing interferons. Although these findings strongly reinforce the concept of acquired immune resistance within the tumour, patients with PD-L1-non-expressing tumours could also potentially benefit from these agents, considering the previously described plasticity of the tumour with the upregulation of PD-L1, the heterogeneity of its expression levels (Taube et al, 2012) and the number of clinical reports where patients with both PD-L1-positive and -negative baseline tumour biopsies were associated with clinical benefit, although the latest group with less objective responses (Hodi et al, 2014; Kefford et al, 2014).
Programmed cell death protein-1/ligand-1 has also been studied as a prognostic biomarker in many different primary tumours, with equivocal results (Rosenwald et al, 2003; Parsa et al, 2007; Hino et al, 2010). Variations in cancer type, stage of cancer analysed, PD-L1 monoclonal antibody used for IHC staining, laboratory techniques for IHC staining and treatment history may have contributed to the diverse results.
Targeting the PD-1 pathway
Several antibodies that inhibit the PD-1 pathway by blocking either PD-1 or PD-L1 are being developed for clinical use in a variety of tumour types and clinical settings (Table 1). These agents differ in structure and are generally classified into two groups: anti-PD1 and anti-PD-L1 antibodies. Antibodies that inhibit PD-1 block its binding to both PD-L1 and PD-L2, whereas anti-PD-L1 antibodies only block the PD-1:PD-L1 interaction and potentially the PD-1:CD80 interaction. Whether this difference affects clinical activity, organ-specific immune modulation or toxicity needs to be elucidated.
Regarding the anti-PD-1 antibodies, the fully human nivolumab (Bristol-Myers Squibb) and the humanised pembrolizumab (Merck, Whitehouse Station, NJ, USA) are IgG4 monoclonal antibodies that block the binding of PD-1 receptor to PD-L1 and PD-L2. Pembrolizumab has an optimised fragment crystallisable (fc) region that minimises antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). In contrast, the third anti-PD-1 antibody with more extensive clinical development pidilizumab (CureTech, Yavne, Israel) is an IgG1 antibody, which confers more ADCC and CDC. Antibodies in clinical development that target PD-L1 include MPDL3280A (Genentech, South San Francisco, CA, USA), MEDI4736 (MedImmune/AstraZeneca) and MSB0010718C (EMD Serono, Rockland, MA, USA), all of them IgG1 isotype. MPDL3280A and MEDI4736 have genetically modified Fc fragments to avoid ADCC, which is of particular importance for anti-PD-L1 antibodies as activated T cells readily express PD-L1 (Herbst et al, 2014). No data are available regarding the comparison of these agents and we will focus on describing the clinical data available for these antibodies in different tumours.
PD-1 blockade efficacy: first evidence of clinical activity
The most extensive clinical experience with PD-1 antibodies has been obtained with both nivolumab and pembrolizumab, which have demonstrated highly durable response rates with acceptable toxicity in large phase I studies involving patients with advanced melanoma, NSCLC, renal cell carcinoma (RCC) and Hodgkin’s disease, among others (Topalian et al, 2012b, 2014; Hamid et al, 2013a; Ansell et al, 2015).
Nivolumab was first evaluated in a phase I/II study in 296 patients with a variety of heavily pretreated malignancies including melanoma, NSCLC, prostate cancer, RCC and colorectal cancer. Patients received nivolumab at doses of 1–10 mg kg−1 of body weight every 2 weeks for up to 12 cycles until disease progression or a complete response occurred. No maximum-tolerated dose (MTD) was defined and only 14% of the patient experienced grade 3/4 drug-related adverse events. Cumulative response rates (all doses) were 18.4% (14 out of 76) among patients with NSCLC, 27.6% (26 out of 94) among patients with melanoma and 27.3% (9 out of 33) among patients with RCC (Topalian et al, 2012b). These results generated enthusiasm among oncologists and provided evidence of clinical activity in neoplasms classically considered non-immunogenic.
In parallel to the clinical development of nivolumab, the anti-PD-1 antibody pembrolizumab was similarly showing impressive tumour responses in a more restricted population of patients with advanced melanoma (Hamid et al, 2013a). Hamid et al (2013a) reported 135 patients with advanced melanoma being treated with three separate dosing strategies: 10 mg kg−1 of body weight every 2 or 3 weeks or 2 mg kg−1 every 3 weeks. Some patients were previously treated with ipilimumab. Adverse events were similar to those found in patients treated with nivolumab, including fatigue, rash, pruritus and diarrhoea. Response rates across all dose levels were 38%, with patients on the highest dose of pembrolizumab showing a response rate of 52%. Responses were durable, and the median progression-free survival (PFS) was longer than 7 months. A subsequent prospective, randomised analysis was performed using both 2 and 10 mg kg−1 doses given every 3 weeks to patients with ipilimumab-refractory advanced melanoma. The response rate was 26% at both doses and the safety profile was similar, making 2 mg kg−1 once every 3 weeks the recommended dose for further studies (Robert et al, 2014). An updated report on the phase I clinical trial (KEYNOTE-001) included the analysis of 411 melanoma patients treated across multiple dose levels. Median OS data was not available, but 1-year OS rate over all dose cohorts was 69% (Ribas et al, 2014a).
Pidilizumab has been more predominantly evaluated in haematologic malignancies. A phase I dose-escalating trial tested pidilizumab in 17 patients with refractory acute myeloid leukaemia, chronic lymphocytic leukaemia, Hodgkin and non-Hodgkin lymphoma or multiple myeloma and reported an acceptable safety profile, with no MTD defined and clinical benefit in 33% of the patients evaluated (Berger et al, 2008).
Targeting PD-L1 is a similarly promising approach to targeting PD-1. BMS-956559, although no longer under clinical development, was the first PD-L1 antibody to show durable tumour regressions in patients with a variety of solid tumours, mostly NSCLC and melanoma, and also RCC, colorectal, ovarian, pancreatic, gastric and breast cancer (Brahmer et al, 2012).
Other anti-PD-L1 antibodies such as MPDL3280A, MEDI4736 and MSB0010718C have also shown responses in early-phase clinical trials in a number of malignancies (Herbst et al, 2013; Heery et al, 2014; Segal et al, 2014). MPDL3280A phase I testing in patients with multiple histologies was well tolerated and did not reach a MTD (Herbst et al, 2013). Pharmacokinetic data supported dosing at 15 mg kg−1 every 3 weeks and activity was observed in multiple tumour types including NSCLC, RCC, melanoma, colorectal and gastric cancer. In phase I, another anti-PD-L1 antibody, MEDI4736, exhibited dose-dependent pharmacokinetics and yielded dose-dependent PD-L1 suppression (Fairman et al, 2014), with an acceptable safety profile, no MTD and evidence of clinical activity across multiple cancer types including melanoma, gastro-oesophageal, pancreatic cancer and head and neck squamous cell carcinoma (Segal et al, 2014). MSB0010718C dose-escalation trial was performed and investigators demonstrated that MSB0010718C could be safely administered in doses up to 20 mg kg−1 2 weeks. Further development of this antibody is planned in patients with metastatic or locally advanced solid tumours. A summary of the phase II/III relevant clinical data for each agent is included in Table 2 and detailed below.
PD-1/L1 blockade in different tumours
Melanoma
Nivolumab was recently compared with dacarbazine in a phase III randomised double blind study in patients with treatment-naive BRAF wild-type advanced melanoma (n=418) (Robert et al, 2014). The median OS was not reached for nivolumab vs 10.8 months for dacarbazine and 1-year survival rate was 73% vs 42%, respectively. This survival advantage was observed in both PD-L1-positive and -negative nivolumab-treated patients. Drug-related adverse events were more common in the dacarbazine-treated group. In a separate phase III trial, nivolumab was compared with investigator’s choice chemotherapy in patients who had experienced progression on ipilimumab and resulted in an increased overall response rate from 11% to 32%, with less frequent high-grade adverse events (Weber et al, 2014). Nivolumab was approved in December 2014 for patients with advanced melanoma after disease progression to a previous therapy.
Phase II randomised clinical data with pembrolizumab have also been recently reported. At the 2014 Society for Melanoma Research (SMR) meeting, results on the primary end point of PFS comparing two dosing regimens of pembrolizumab with chemotherapy in patients with ipilimumab-refractory advanced melanoma were presented (Ribas et al, 2014b). Pembrolizumab provided significant improvements in PFS and durable objective responses while better preserving health-related quality of life compared with chemotherapy. Pembrolizumab received FDA approval in September 2014 for patients with melanoma previously treated with ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor.
Expansion cohorts of patients with metastatic melanoma treated with MPDL3280A resulted in an overall response rate of 26% (9 out of 35) and a 24-week PFS of 35% (Hamid et al, 2013b). MPDL3280A is currently being explored in combination with vemurafenib in advanced BRAF-mutated melanoma. The anti-PD-L1 antibody MEDI4736 is also currently under investigation in combination with dabrafenib and trametinib, or with trametinib alone, in subjects with metastatic melanoma with or without BRAF mutations, respectively.
Targeting T-cell activation at different stages of the immune response might lead to an increased efficacy in the clinical setting, while potentially delaying resistance to either agent. Combining the blockade of PD-1 and CTLA-4 in preclinical models achieved a more pronounced antitumour activity than blockade of either pathway alone and provided the rationale for further studying this combination (Curran et al, 2010). A phase I study evaluated the safety and efficacy of a concurrent regimen of ipilimumab and nivolumab in patients with advanced melanoma (Wolchok et al, 2013). Concurrent treatment was associated with an overall response rate of 40%, which seemingly exceeded the previously reported results with either agent alone (Hodi et al, 2010; Topalian et al, 2012b). However, 53% of the patients treated with the concurrent regimen had grade 3/4 treatment-related adverse events, which is higher than the previous rates among patients treated with ipilimumab or nivolumab alone. Whether the antitumour effect of both agents is more active than either agent alone is being addressed in a phase III clinical trial and results are eagerly awaited.
Non-small-cell lung carcinoma
Non-small-cell lung carcinoma is one great example of a cancer type that was not believed to be immune-responsive and where antibodies blocking the PD-1 checkpoint have shown therapeutic activity. Similar to melanoma, expression of immunoinhibitory molecules in the tumour microenvironment appears to be a relevant mechanism for immune resistance in NSCLC. Both PD-L1 and PD-L2 have been reported to be upregulated in NSCLC (Zou and Chen, 2008; Pardoll, 2012). Downregulation of components of the antigen-presenting machinery, particularly among smoking-associated lung cancer patients (Pleasance et al, 2010), appears to be another important immune resistance mechanism.
The initial signs of anti-PD-1 activity in patients with NSCLC were reported in the dose-escalation phase I clinical trial that evaluated nivolumab in different solid tumours (Topalian et al, 2012b). Expansion cohorts of patients with NSCLC showed significant responses and disease stabilisation in both non-squamous and squamous lung carcinoma. Durability of clinical responses, unique to immunotherapy, was unprecedented (median duration of response was 74 weeks and 1- and 2-year OS was 42% and 14%, respectively) (Brahmer et al, 2013). Of note, eight patients developed pneumonitis, three of which were grade 3/4. This organ-specific immune toxicity might be of particular importance in patients with NSCLC, as these patients may harbor some degree of pulmonary inflammation that might be enhanced upon PD-1 blockade.
Taken together, these encouraging results have led to the development of nivolumab in two separate phase III clinical trials. As a single agent, nivolumab is being compared with docetaxel in patients with non-squamous histology in the second- or third-line treatment setting and in patients with squamous cell histology after one prior platinum-containing regimen. In addition to multiple treatment-arm phase I trials where this antibody is being combined with various chemotherapy regimens, nivolumab is also being explored in the neoadjuvant setting in resectable NSCLC.
Regarding the testing of pembrolizumab in this patient population, a phase I clinical trial of previously treated patients with locally advanced or metastatic NSCLC was also recently reported. Enrolled patients had PD-L1 detected in their tumours by an immunohistochemical assay, although some patients with tumours without PD-L1 expression who had received at least two prior lines of therapy were also included. Treatment was generally well tolerated, although three patients experienced grade 3/4 drug-related pneumonitis. Robust antitumour activity was observed with a preliminary higher response rate (24%) for patients with PD-L1-positive tumours compared with PD-L1-non-expressing tumours (8%) (Garon et al, 2014).
Blocking anti-PD-L1 is a strategy extensively being evaluated in patients with NSCLC. MPDL3280A expansion cohort for previously treated patients with metastatic NSCLC observed an overall response rate of 24% (9 out of 37) in patients with both squamous and non-squamous histology, including several patients with rapid tumour shrinkage and additional patients with delayed responses after apparent radiographic progression (Spigel et al, 2013). There were no cases of grade 3/4 pneumonitis, suggesting a potentially more tolerable safety profile with anti-PD-L1 inhibitors in patients with NSCLC. Ongoing trials include a single-arm phase II study of MPDL3280A in chemonaive and prior platinum-based treated patients with PD-L1-positive tumours and a phase III efficacy and safety evaluation of MPDL3280A compared with docetaxel after failure of a platinum-containing chemotherapy regimen.
Preliminary clinical activity with MEDI4736 in patients with NSCLC has also been observed, with three partial responses and two additional patients showing tumour shrinkage not meeting partial response out of 13 patients evaluated (Brahmer et al, 2014). Same as with MPDL3280A, safety was acceptable and no grade 3/4 pneumonitis was observed. Several large combination trials are ongoing including the combination of an anti-EGFR and MEDI4736 in advanced NSCLC, its sequential administration after concurrent chemoradiation in stage III or vs placebo after a complete resection.
Renal cell carcinoma
Immunomodulation has classically been considered a therapeutic strategy for RCC, and cytokine-based immunotherapeutic agents such as IL-2 are associated with modest rates of highly durable responses. PD-L1 is increased in inflammatory conditions of the kidney and in RCC, as opposed to normal renal tissue, suggesting its role in negatively regulating T-cell function (Ding et al, 2005).
A randomised phase II clinical trial evaluated different doses of the nivolumab in patients with advanced RCC and observed long-lasting objective responses in 20–22% of the patients evaluated across all groups. Median OS was 18.2 months for the 0.3 mg kg−1 dose and was not reached for the 2 or 10 mg kg−1 doses (Motzer et al, 2014a). Results from a phase III study comparing nivolumab to everolimus in pretreated metastatic RCC could potentially lead to the registration of the anti-PD-1 antibody in this therapeutic setting. Nivolumab is currently being developed in combination with either sunitinib or pazopanib, with promising results in terms of efficacy but high level of toxicity (Amin et al, 2014). In the same trial, two separate arms evaluated the combination of ipilimumab plus nivolumab, with preliminary results suggesting the synergy of the combination, at the expense of significant toxicity (Hammers et al, 2014).
Pembrolizumab is currently being investigated in a phase I/II trial in combination with pazopanib in treatment-naive patients with metastatic RCC. Once the recommended phase II dose is determined, the potential for synergy combining both agents will be evaluated. Other antiangiogenics combined with pembrolizumab include axitinib.
The initial experience with MPDL3280A in RCC indicated the presence of responses across all dose, with some patients with RCC experiencing prolonged stable disease before experiencing tumour response. The 24-week PFS was 50% among the 39 patients evaluated for efficacy (Cho et al, 2013). MPDL3280A is currently being investigated as monotherapy or in combination with bevacizumab as compared with a control arm of sunitinib in patients with treatment-naive, locally advanced or metastatic RCC.
Triple-negative breast cancer
Programmed cell death protein-1/ligand-1 is expressed in 20% of triple-negative breast cancer (TNBCs), suggesting PD-L1 as a therapeutic target in TNBCs (Mittendorf et al, 2014). A phase Ib clinical trial with pembrolizumab in 27 patients with heavily pretreated recurrent or metastatic TNBC positive for PD-L1 achieved one complete response, four partial responses and seven cases with stable disease. Another complete response and two partial responses were obtained in a similar subgroup of patients with MPDL3280A.
Bladder cancer
A clinical trial involving patients with heavily pretreated metastatic bladder cancer selected to be PD-L1-positive based on an analysis of the PD-L1 expression on immune-infiltrating cells resulted in 10 out of 20 with a response (9 partial responses and 1 complete response). Response rates for patients with PD-L1-negative tumours have yet to be published (Powles et al, 2014). This study had a short follow-up of just 2.8 months, and further information will be needed to assess the benefits of PD-1/L1 blockade in patients with bladder cancer.
Haematologic malignancies
The importance of the PD-1 axis in leukaemias and lymphomas has been demonstrated in several different scenarios. Examples of haematologic malignancies that frequently express PD-L1 include adult T-cell leukaemia lymphoma (Kozako et al, 2009), angioimmunoblastic T-cell lymphoma (Xerri et al, 2008, Wilcox et al, 2009) and non-Hodgkin lymphoma (Andorsky et al, 2011), among others.
Phase II clinical trial combining pidilizumab and rituximab in patients with relapsed follicular lymphoma demonstrated an overall response rate of 66%, with 52% of participants achieving a complete response. No grade 3/4 side effects were observed (Westin et al, 2010). Patients with heavily pretreated relapsing or refractory classic Hodgkin lymphoma were recently included as an independent cohort in a dose escalation and cohort expansion phase I study of nivolumab (Armand et al, 2014). With an objective response rate as high as 87% (20 out of 23) including 17% complete responses and a PFS rate at 24 weeks of 86%, a phase II trial of nivolumab in this subset of patients is already underway.
Patients with relapsed or refractory classical Hodgkin lymphoma were evaluated as a cohort of the ongoing multicentre, open-label, phase Ib clinical trial of pembrolizumab in haematologic malignancies and patients were treated with single-agent pembrolizumab 10 mg kg−1 administered intravenously every 2 weeks. Twenty per cent of the 15 evaluable patients had a CR at 12 weeks. Additionally, 33% had partial remission as best overall response, for an overall response rate of 53% (Moskowitz et al, 2014).
Conclusion
Anti-PD-1/L-1 antibodies are revitalising interest in solid tumour immunotherapy after demonstrating impressive rates of clinical benefit in patients with different neoplasms, some of them classically not considered immunoresponsive. We are starting to understand which patients are more likely to benefit from anti-PD-1/L1-blocking strategies, and the potential for biomarkers associated with response will ultimately lead to improved patient care. Because of the complexity of the tumour environment, the high number of cells and molecules implicated in tumour immune evasion and therefore potential therapeutic targets, further studies might likely uncover additional immunologic checkpoints, which can be targeted alone or in combination with other immunotherapeutic approaches. Such combinations will need to be developed under a strong preclinical rational and taking into consideration the differences in the development of immunotherapeutic agents compared with classic anticancer agents.
References
Amin A, Plimack ER, Infante JR, Ernstoff MS, Rini BI, Mcdermott DF, Knox JJ, Pal SK, Voss MH, Sharma P, Kollmannsberger CK, Heng DY, Spratlin JL, Shen Y, Kurland JF, Gagnier P, Hammers HH (2014) Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC) [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM (2011) Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res 17: 4232–4244.
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372: 311–319.
Armand P, Ansell SM, Lesokhin AM, Halwani A, Millenson MM, Schuster SJ, Timmerman J, Borrello I, Gutierrez M, Scott EC, Cattry D, Chapuy B, Ligon AH, Rodig SJ, Zhu L, Grosso JF, Kim SY, Shipp MA (2014) Preliminary Results of a Phase I Study of Nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies [abstract]. Am Soc Hematol (Meeting Abstracts) 289.
Atkins MB, Kudchadkar RR, Sznol M, Mcdermott MD, Lotem M, Schachter J, Wolchok JD, Urba WJ, Kuzel T, Schuchter LM, Slingluff CL, Ernstoff MS, Fay JW, Friedlander PA, Gajewski T, Zarour HM, Rotem-Yehudar R, Sosman JA (2014) Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A (2008) Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14: 3044–3051.
Brahmer JR, Horn L, Antonia SJ, Spigel DR, Gandhi L, Sequist LV, Sankar V, Ahlers CM, Wigginton JM, Kollia G, Gupta AK, Gettinge SN (2013) Survival and long-term follow-up of the phase I trial of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with previously treated advanced non-small cell lung cancer [abstract]. J Clin Oncol (Meeting Abstracts) 31: abstract 8030.
Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A, Li X, Robbins PB, Vasselli J, Ibrahim RA, Antonia SJ (2014) Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Brahmer JR, Tykodi SS, Chow LQM, Hwu W, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465.
Cho DC, Sosman JA, Sznol M, Gordon MS, Hollebecque A, Hamid O, Mcdermott DF, Delord J-P, Rhee IP, Mokatrin A, Kowanetz M, Funke RP, Fine GD, Powles T (2013) Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) [abstract]. J Clin Oncol (Meeting Abstracts) 31: abstract 4505.
Curran MA, Montalvo W, Yagita H, Allison JP (2010) PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA 107: 4275–4280.
Ding H, Wu X, Gao W (2005) PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol 115: 184–191.
Egen JG, Allison JP (2002) Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16: 23–35.
Fairman D, Narwal R, Liang M, Robbins PB, Schneider A, Chavez C, Lu H, Pak M, Blake-Haskins A, Vasselli J, Ibrahim RA, Shalabi AM, Roskos L (2014) Pharmacokinetics of MEDI4736, a fully human anti-PDL1 monoclonal antibody, in patients with advanced solid tumors [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206: 3015–3029.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T (2000) Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192: 1027–1034.
Garon EB, Leighl NB, Rizvi NA, Blumenschein GR, Balmanoukian AS, Eder JP, Goldman JW, Hui R, Soria J, Gangadhar TC, Sun J, Patnaik A, Gubens MA, Lubiniecki GM, Zhang J, Niewood M, Emancipator K, Dolled-Filhart M, Hanson ME, Gandhi L (2014) Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A (2013a) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369: 134–144.
Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, Boasberg PD, Flaherty K, Hwu P, Ballinger M, Mokatrin A, Kowanetz M, Chen DS, Hodi FS (2013b) Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) [abstract]. J Clin Oncol (Meeting Abstracts) 31: abstract 9010.
Hammers H, Plimack E, Infante J, Ernstoff M, Rini B, Mcdermott D, Albiruni R, Razak A, Pal S, Voss M, Sharma P, Kollmannsberger C, Heng D, Spratlin J, Shen Y, Kurland J, Gagnier G, Amin A (2014) Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC). J Clin Oncol 32: 5s.
Heery CR, Coyne GS, Madan RA, Schlom A, Heydebreck A, Cuillerot J, Sabzevari H, Gulley JL (2014) Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Herbst RS, Gordon MS, Fine GD, Sosman JA, Soria J, Hamid O, Powderly JD, Burris HA, Mokatrin A, Kowanetz M, Leabman M, Anderson M, Chen DS, Hodi FS (2013) A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors [abstract]. J Clin Oncol (Meeting Abstracts) 31: abstract 3000.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, Mcdermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515: 563–567.
Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y (2010) Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 116: 1757–1766.
Hodi FS, O’day SJ, Mcdermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, Van Den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723.
Hodi FS, Sznol M, Kluger HM, Mcdermott DF, Carvajal RD, Lawrence DP, Topalian SL, Atkins MB, Powderly JD, Sharfman WH, Puzanov I, Smith DC, Leming PD EJL, Taube JM, Anders R, Horak CE, Kollia G, Gupta AK, Sosman JA (2014) Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5.
Ishida Y, Agata Y, Shibahara K, Honjo T (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11: 3887–3895.
Kefford R, Ribas A, Hamid O, Robert C, Daud A, Wolchok JD, Joshua AM, Hodi FS, Gangadhar TC, Hersey P, Weber JS, Dronca RS, Patnaik A, Zarour HM, Dolled-Filhart M, Lunceford J, Emancipator K, Ebbinghaus S, Kang SP, Hwu W (2014) Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475 [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH (2006) Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203: 883–895.
Korman AJ, Peggs KS, Allison JP (2006) Checkpoint blockade in cancer immunotherapy. Adv Immunol 90: 297–339.
Kozako T, Yoshimitsu M, Fujiwara H, Masamoto I, Horai S, White Y, Akimoto M, Suzuki S, Matsushita K, Uozumi K, Tei C, Arima N (2009) PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia 23: 375–382.
Lenschow DJ, Walunas TL, Bluestone JA (1996) CD28/B7 system of T cell costimulation. Annu Rev Immunol 14: 233–258.
Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R (1994) Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity 1: 793–801.
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, Chawla A, Curran M, Hwu P, Sharma P, Litton JK, Molldrem JJ, Alatrash G (2014) PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2: 361–370.
Moskowitz CH, Ribrag V, Michot J, Martinelli G, Zinzani PL, Gutierrez M, De Maeyer G, Jacob AG, Giallella K, Anderson JW, Derosier MD, Wang J, Yang Z, Rubin R, Rose S, Shipp MA, Armand P (2014) 290 PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013) [abstract]. Am Soc Hematol (Meeting Abstracts) 290.
Motzer R, Rini B, Mcdermott D, Redman B, Kuzel T, Harrison M, Ua V, Drabkin H, George S, Logan T, Margolin K, Plimack E, Waxman I, Lambert A, Hj H (2014a) Nivolumab for metastatic renal cell carcinoma (mRCC): Results of a randomized, dose-ranging phase II trial. J Clin Oncol (Meeting Abstracts) 32: 5s.
Motzer RJ, Rini BI, Mcdermott DV, Redman BG, Kuzel T, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack ER, Waxman I, Lambert A, Hammers HJ (2014b) Nivolumab for metastatic renal cell carcinoma (mRCC): results of a randomized, dose-ranging phase II trial [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Okazaki T, Honjo T (2007) PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol 19: 813–824.
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12: 252–264.
Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13: 84–88.
Pleasance ED, Stephens PJ, O’meara S, Mcbride DJ, Meynert A, Jones D, Lin ML, Beare D, Lau KW, Greenman C, Varela I, Nik-Zainal S, Davies HR, Ordonez GR, Mudie LJ, Latimer C, Edkins S, Stebbings L, Chen L, Jia M, Leroy C, Marshall J, Menzies A, Butler A, Teague JW, Mangion J, Sun YA, Mclaughlin SF, Peckham HE, Tsung EF, Costa GL, Lee CC, Minna JD, Gazdar A, Birney E, Rhodes MD, Mckernan KJ, Stratton MR, Futreal PA, Campbell PJ (2010) A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 463: 184–190.
Powles T, Vogelzang NJ, Fine GD, Eder JP, Braiteh FS, Loriot Y, Zambrano CC, Bellmunt J, Burris HA, Teng SM, Shen X, Koeppen H, Hegde PS, Chen DS, Petrylak DP (2014) Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Ribas A, Hodi FS, Kefford R, Hamid O, Daud A, Wolchok JD, Hwu W, Gangadhar TC, Patnaik A, Joshua AM, Hersey P, Weber JS, Dronca RS, Zarour HM, Gergich K, Li X, Iannone R, Kang SP, Ebbinghaus S, Robert C (2014a) Efficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL) [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Ribas A, Tumeh PC (2014) The future of cancer therapy: selecting patients likely to respond to PD1/L1 blockade. Clin Cancer Res 20: 4982–4984.
Ribas A, Wolchok JD, Robert C, Kefford K, Hamid O, Daud A, Hwu WJ, Weber JS, Joshua AM, Gangadhar TC, Patnaik A, Hersey P, Dronca R, Zarour H, Gergich K, Lindia JA, Giannotti M, Li X, Ebbinghaus S, Kang SP, Hodi FS (2014b) Updated clinical efficacy of the anti-PD-1 monoclonal antibody pembrolizumab (pembro, MK-3475) in 411 patients (pts) with melanoma (MEL) [abstract]. Soc Melanoma Res (Meeting Abstracts).
Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS (2002) Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA 99: 11790–11795.
Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC, Patnaik A, Dronca R, Zarour H, Joseph RW, Boasberg P, Chmielowski B, Mateus C, Postow MA, Gergich K, Elassaiss-Schaap J, Li XN, Iannone R, Ebbinghaus SW, Kang SP, Daud A (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384: 1109–1117.
Robert C, Thomas L, Bondarenko I, O’day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364: 2517–2526.
Robinson MR, Chan CC, Yang JC, Rubin BI, Gracia GJ, Sen HN, Csaky KG, Rosenberg SA (2004) Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother 27: 478–479.
Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, Chan WC, Zhao T, Haioun C, Greiner TC, Weisenburger DD, Lynch JC, Vose J, Armitage JO, Smeland EB, Kvaloy S, Holte H, Delabie J, Campo E, Montserrat E, Lopez-Guillermo A, Ott G, Muller-Hermelink HK, Connors JM, Braziel R, Grogan TM, Fisher RI, Miller TP, Leblanc M, Chiorazzi M, Zhao H, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Staudt LM (2003) Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 198: 851–862.
Segal NH, Antonia SJ, Brahmer JR, Maio M, Blake-Haskins A, Li X, Vasselli J, Ibrahim RA, Lutzky J, Khleif S (2014) Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody [abstract]. J Clin Oncol (Meeting Abstracts) 32: 5s.
Spigel DR, Gettinger SN, Horn L, Herbst RS, Gandhi L, Gordon MS, Cruz C, Conkling P, Cassier PA, Antonia SJ, Burris HA, Fine GD, Mokatrin A, Kowanetz M, Shen X, Chen DS, Soria J (2013) Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol (Meeting Abstracts) 31: abstract 8008.
Taube JM, Anders RA, Young GD, Xu H, Sharma R, Mcmiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4: 127ra137.
Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G, Prevost-Blondel A, Kato M, Schultze JL, Tartour E, Kroemer G, Chaput N, Zitvogel L (2011) IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 71: 5393–5399.
Topalian SL, Drake CG, Pardoll DM (2012a) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24: 207–212.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, Mcdermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, PD Leming, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, Mcmiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, Mcdonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012b) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454.
Topalian SL, Sznol M, Mcdermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32: 1020–1030.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515: 568–571.
Weber JS, Minor DR, D’angelo S, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH, Grob J, Lao C, Linette G, Grossman K, Hassel J, Lorigan P, Maio M, Snolz M, Lambert A, Yang A, Larkin J (2014) A phase 3 randomized, open-label study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) versus investigator’s choice chemotherapy (ICC) in patients with advanced melanoma after prior anti-CTLA-4 therapy [abstract]. Eur Soc Med Oncol Congr (Meeting Abstracts) 728.
Westin J, Chu F, Foglietta M, Rotem-Yehudar R, Neelapu S (2010) Phase II safety and efficacy study of CT-011, a humanized anti-PD-1 monoclonal antibody, in combination with rituximab in patients with relapsed follicular lymphoma. J Clin Oncol (Meeting Abstracts) 28: 15s.
Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, Dong H, Kwon ED, Novak AJ, Markovic SN, Pittelkow MR, Witzig TE, Ansell SM (2009) B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 114: 2149–2158.
Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322: 271–275.
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369: 122–133.
Xerri L, Chetaille B, Serriari N, Attias C, Guillaume Y, Arnoulet C, Olive D (2008) Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol 39: 1050–1058.
Zou W, Chen L (2008) Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 8: 467–477.
Acknowledgements
This work was supported by NIH Grants P01 CA168585, P01 CA132681, P50 CA086306, R01 CA199205, the Dr Robert Vigen memorial fund, the Ressler Family Foundation, the Louise Belley and Richard Schnarr Fund, the Wesley Coyle Memorial Fund and the Garcia-Corsini Family Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This work is licensed under the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Homet Moreno, B., Ribas, A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer 112, 1421–1427 (2015). https://doi.org/10.1038/bjc.2015.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2015.124
This article is cited by
-
Natural products and the balancing act of autophagy-dependent/independent ferroptosis in cancer therapy
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Exosomes in the tumor microenvironment of cholangiocarcinoma: current status and future perspectives
Journal of Translational Medicine (2022)
-
Cognitive assessment in patients treated by immunotherapy: the prospective Cog-Immuno trial
BMC Cancer (2022)
-
Discovery and pharmacological characterization of cetrelimab (JNJ-63723283), an anti–programmed cell death protein-1 (PD-1) antibody, in human cancer models
Cancer Chemotherapy and Pharmacology (2022)
-
Construction of an N6-methyladenosine lncRNA- and immune cell infiltration-related prognostic model in colorectal cancer
Protoplasma (2022)