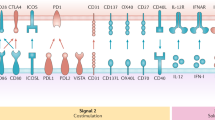
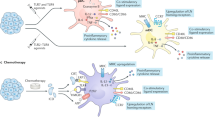
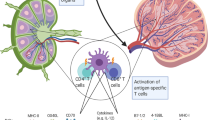
Abstract
Dendritic cells, the most potent ‘professional’ antigen-presenting cells, hold promise for improving the immunotherapy of cancer. In three different well-characterized tumour models, naive mice injected with bone marrow-derived dendritic cells prepulsed with tumour-associated peptides previously characterized as being recognized by class I major histocompatibility complex-restricted cytotoxic T lymphocytes, developed a specific T-lymphocyte response and were protected against a subsequent lethal tumour challenge. Moreover, in the C3 sarcoma and the 3LL lung carcinoma murine models, treatment of animals bearing established macroscopic tumours (up to 1 cm2 in size) with tumour peptide-pulsed dendritic cells resulted in sustained tumour regression and tumour-free status in more than 80% of cases. These results support the clinical use of tumour peptide-pulsed dendritic cells as components in developing effective cancer vaccines and therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pardoll, D. Tumour antigens: A new look for the 1990s. Nature 369, 357–358 (1994).
Grabbe, S., Beissert, S., Schwartz, T. & Granstein, R.D. Dendritic ceils as initiators of tumor immune responses: A possible strategy for tumor immunotherapy? Immun. Today 16, 116–120 (1995).
Tamaki, K., Stingl, G., Gullino, M., Sachs, D.H. & Katz, S.I. Ia antigens in mouse skin are predominantly expressed on Langerhans cells. J. Immun. 123, 784–787 (1979).
Razi-Wolf, Z., Falo, L.D. & Reiser, H. Expression and function of the costimulatory molecule B7 on murine Langerhans cells: Evidence for an alternative CTLA-4 ligand. Eur. J. Immun. 24, 805–811 (1994).
Inaba, K. et al. Identification of proliferating dendritic cell progenitors in mouse blood. J. exp. Med. 175, 1157–1167 (1992).
Inaba, K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. exp. Med. 176, 1693–1702 (1992).
Caux, C., Dezutter-Dambuyant, C., Schmitt, D. & Banchereau, J. GM-CSF and TNFa cooperate in the generation of dendritic Langerhans cells. Nature 360, 258–261 (1992).
Caux, C. et al. Potentiation of early hematopoiesis by TNF alpha is followed by inhibition of granulopoietic differentiation and proliferation. Blood 78, 635–644 (1991).
Koch, F. et al. Tumor necrosis factor a maintains the viability of murine epidermal Langerhans cells in culture, but in contrast to granulocyte/macrophage colony-stimulating factor, without inducing their functional maturation. J. exp. Med. 171, 159–171 (1990).
Romani, N. et al. Proliferating dendritic cell progenitors in human blood. J. exp. Med. 180, 83–93 (1994).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin-4 and downregulated by tumor necrosis factor alpha. J. exp. Med. 179, 1109–1118 (1994).
Zorina, T. et al. Culture of dendritic cells from murine bone marrow supplemented with GM-CSF and TNF-alpha. J. Immunother. 16, 247 (1994).
Lanier, L.L. et al. CD80(B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J. Immun. 154, 97–105 (1995).
Caux, C. et al. B70/B7-2 is identical to CD86 and is the major functional ligand for CD28 expressed on human dendritic cells. J. exp. Med. 180, 1841–1847 (1994).
Mandelboim, O. et al. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature 369, 67–71 (1994).
Feltkamp, M.C.W. et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur. J. Immun. 23, 2242–2249 (1993).
Falk, K., Rotzschke, O., Stevanovic, S., Jung, G. & Rammensee, H.-G. Allele-specific models revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351, 290–296 (1991).
Falo, L.D., Kovacsovics-Bankowski, M., Thompson, K. & Rock, K.L. Targeting antigen into the phagocytic pathway In vivo induces protective tumour immunity. Nature Med. 1, 649–653 (1995).
Porgador, A. & Gilboa, E. Bone marrow-generated dendritic cells pulsed with a class I-restricted peptide are potent inducers of cytotoxic T lymphocytes. J. exp. Med. 182, 255–260 (1995).
Gyure, L.A., Barfoot, R., Denham, S. & Hall, J.G. Immunity to a syngeneic sarcoma induced in rats by syngeneic lymph cells exposed to the tumour either in vivo or in vitro. Br. J. Cancer 55, 17–20 (1987).
Knight, S.C., Hunt, R., Dote, C. & Medawar, P.B. Influence of dendritic cells on tumor growth. Proc. natn. Acad. Sci. U.S.A. 82, 4495–4497 (1985).
Caux, C., Liu, Y-L. & Banchereau, J. Recent advances in the study of dendritic cells and follicular dendritic cells. Immun. Today 16, 2–4 (1995).
Grabbe, S. et al. Tumor antigen presentation by murine epidermal cells. J. Immun. 146, 3656–3661 (1991).
Flamand, V. et al. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur. J. Immun. 24, 605–610 (1994).
Hsu, F.J. et al. Antigen-pulsed dendritic cells are effective in inducing immune responses in patients with B-cell lymphoma. Blood 84, 520a (1994).
Kast, W.M., Boog, C.J.P., Roerp, B.O., Voordouw, A.C. & Melief, C.J.P. Failure or success in the restoration of virus-specific cytotoxic T lymphocyte response defects by dendritic cells. J. Immun. 140, 3186–3193 (1988).
Mizoguchi, H. et al. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science 258, 1795–1798 (1992).
Pan, Z.-K., Ikonomidis, G., Lazenby, A., Pardoll, D. & Paterson, Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nature Med. 1, 471–477 (1995).
Aichele, P., Brduscha-Riem, K., Zinkernagel, R.M., Hengartner, H. & Pircher, H. T cell priming versus T cell tolerance induced by synthetic peptides. J. exp. Med. 182, 261–266 (1995).
Jenkins, M.K. The ups and downs of T cell costimulation. Immunity 1, 443–446 (1994).
Bellone, M. et al. In vitro priming of cytotoxic T lymphocytes against poorly immunogenic epitopes by engineered antigen-presenting cells. Eur. J. Immun. 24, 2691–2698 (1994).
Frassanito, M.A. et al. Identification of Meth A sarcoma-derived class I major histocompatibility complex-associated peptides recognized by a specific CD8+ cytotoxic T lymphocyte. Cancer Res. 55, 124–128 (1995).
Storkus, W.J. & Lotze, M.T. Biology of tumor antigens: Tumor antigens recognized by immune cells. In: Biologic Therapy of Cancer, 2nd edn (eds DeVita, V. T., Hellman, S. & Rosenberg, S.A.) 64–77 (Lippincott, Philadelphia, 1995).
Peoples, G.E. et al. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc. natn. Acad. Sci. U.S.A. 92, 432–436 (1995).
Bosch, F.X. et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. natn. Cancer Inst. 87, 796–802 (1995).
Moore, M.W., Carbone, F.R. & Bevan, M.J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54, 777–785 (1988).
Takahashi, H., Nakagawa, Y., Yokomuro, K. & Berzofsky, J.A. Induction of CD8+ cytotoxic T lymphocytes by immunization with syngeneic irradiated HIV-1 envelope derived peptide-pulsed dendritic cells. Int. Immun. 5, 849–857 (1993).
Rock, K.L., Fleischacker, C. & Gamble, S. Peptide-priming of cytolytic T cell immunity in vivo using (β2-microglobulin as an adjuvant. J. Immun. 150, 1244–1252 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mayordomo, J., Zorina, T., Storkus, W. et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med 1, 1297–1302 (1995). https://doi.org/10.1038/nm1295-1297
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm1295-1297
This article is cited by
-
A novel “prime and pull” strategy mediated by the combination of two dendritic cell-targeting designs induced protective lung tissue-resident memory T cells against H1N1 influenza virus challenge
Journal of Nanobiotechnology (2023)
-
Immune-based therapies in the management of multiple myeloma
Blood Cancer Journal (2020)
-
Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination
Nature Medicine (2019)
-
Improving immune–vascular crosstalk for cancer immunotherapy
Nature Reviews Immunology (2018)
-
Promoting the accumulation of tumor-specific T cells in tumor tissues by dendritic cell vaccines and chemokine-modulating agents
Nature Protocols (2018)