Key Points
-
Apoptosis is a multi-step, multi-pathway cell-death programme that is inherent in every cell of the body. In cancer, the apoptosis:cell-division ratio is altered, which results in a net gain of malignant tissue.
-
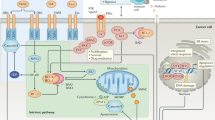
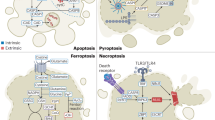
Apoptosis can be initiated either through the death-receptor or the mitochondrial pathway. Caspases that cleave cellular substrates leading to characteristic biochemical and morphological changes are activated in both pathways. The apoptotic process is tightly controlled by various proteins. There are also other caspase-independent types of cell death.
-
Many physiological growth-control mechanisms that govern cell proliferation and tissue homeostasis are linked to apoptosis. Therefore, resistance of tumour cells to apoptosis might be an essential feature of cancer development.
-
Immune cells (T cells and natural killer cells) can kill tumour cells using the granule exocytosis pathway or the death-receptor pathway. Apoptosis resistance of tumour cells might lead to escape from immunosurveillance and might influence the efficacy of immunotherapy.
-
Cancer treatment by chemotherapy and γ-irradiation kills target cells primarily by inducing apoptosis. Therefore, modulation of the key elements of apoptosis signalling directly influences therapy-induced tumour-cell death.
-
Tumour cells can acquire resistance to apoptosis by the expression of anti-apoptotic proteins or by the downregulation or mutation of pro-apoptotic proteins.
-
Alterations of the p53 pathway also influence the sensitivity of tumour cells to apoptosis. Moreover, most tumours are independent of survival signals because they have upregulated the phosphatidylinositol 3-kinase (PI3K)/AKT pathway.
-
The present aim of research in this area is to understand further the molecular mechanisms of tumour resistance and sensitivity, and to use this insight to resensitize tumour cells to apoptosis and, accordingly, to tumour therapy.
Abstract
Every cell in a multicellular organism has the potential to die by apoptosis, but tumour cells often have faulty apoptotic pathways. These defects not only increase tumour mass, but also render the tumour resistant to therapy. So, what are the molecular mechanisms of tumour resistance to apoptosis and how can we use this knowledge to resensitize tumour cells to cancer therapy?
This is a preview of subscription content, access via your institution
Access options





Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Krammer, P. H., Galle, P. R., Moller, P. & Debatin, K. M. CD95(APO-1/Fas)-mediated apoptosis in normal and malignant liver, colon, and hematopoietic cells. Adv. Cancer Res. 75, 251–273 (1998).
Krammer, P. H. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv. Immunol. 71, 163–210 (1999).
Schmitz, I., Kirchhoff, S. & Krammer, P. H. Regulation of death receptor-mediated apoptosis pathways. Int. J. Biochem. Cell Biol. 32, 1123–1136 (2000).
Pitti, R. M. et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396, 699–703 (1998).Identification of the decoy receptor DcR3 that inhibits CD95L-induced apoptosis. DcR3 is amplified in tumours.
Yu, K. Y. et al. A newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J. Biol. Chem. 274, 13733–13736 (1999).
Sprick, M. R. et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12, 599–609 (2000).
Kischkel, F. C. et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 14, 5579–5588 (1995).The first description of the death-inducing signalling complex (DISC) in death receptor-mediated apoptosis.
Kischkel, F. C. et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J. Biol. Chem. 276, 46639–46646 (2001).
Scaffidi, C. et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17, 1675–1687 (1998).Clarifies the role of mitochondria in death-receptor-mediated apoptosis.
Zamzami, N. & Kroemer, G. The mitochondrion in apoptosis: how Pandora's box opens. Nature Rev. Mol. Cell Biol. 2, 67–71 (2001).
Martinou, J. C. & Green, D. R. Breaking the mitochondrial barrier. Nature Rev. Mol. Cell Biol. 2, 63–67 (2001).
Rathmell, J. C. & Thompson, C. B. The central effectors of cell death in the immune system. Annu. Rev. Immunol. 17, 781–828 (1999).
Savill, J. & Fadok, V. Corpse clearance defines the meaning of cell death. Nature 407, 784–788 (2000).
Borner, C. & Monney, L. Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ. 6, 497–507 (1999).
Xiang, J., Chao, D. T. & Korsmeyer, S. J. BAX-induced cell death may not require interleukin 1 β-converting enzyme-like proteases. Proc. Natl Acad. Sci. USA 93, 14559–14563 (1996).
Sperandio, S., de Belle, I. & Bredesen, D. E. An alternative, nonapoptotic form of programmed cell death. Proc. Natl Acad. Sci. USA 97, 14376–14381 (2000).
Krueger, A., Baumann, S., Krammer, P. H. & Kirchhoff, S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 21, 8247–8254 (2001).
Deveraux, Q. L. & Reed, J. C. IAP family proteins — suppressors of apoptosis. Genes Dev. 13, 239–252 (1999).
Verhagen, A. M. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102, 43–53 (2000).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Evan, G. I. & Vousden, K. H. Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348 (2001).
Evan, G. I. et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell 69, 119–128 (1992).
Bissonnette, R. P., Echeverri, F., Mahboubi, A. & Green, D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature 359, 552–554 (1992).References 24 and 69–73 showed that anti-apoptotic proteins are important oncogenes by reporting the oncogenic potential of BCL2.
Harrington, E. A., Bennett, M. R., Fanidi, A. & Evan, G. I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 13, 3286–3295 (1994).
Sabbatini, P., Lin, J., Levine, A. J. & White, E. Essential role for p53-mediated transcription in E1A-induced apoptosis. Genes Dev. 9, 2184–2192 (1995).
Stambolic, V., Mak, T. W. & Woodgett, J. R. Modulation of cellular apoptotic potential: contributions to oncogenesis. Oncogene 18, 6094–6103 (1999).
Datta, S. R., Brunet, A. & Greenberg, M. E. Cellular survival: a play in three Akts. Genes Dev. 13, 2905–2927 (1999).
Frisch, S. M. & Screaton, R. A. Anoikis mechanisms. Curr. Opin. Cell Biol. 13, 555–562 (2001).
Frisch, S. M. & Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626 (1994).
Khwaja, A., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H. & Downward, J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16, 2783–2793 (1997).
Puthalakath, H., Huang, D. C., O'Reilly, L. A., King, S. M. & Strasser, A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3, 287–296 (1999).
Puthalakath, H. et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293, 1829–1832 (2001).
Oulton, R. & Harrington, L. Telomeres, telomerase, and cancer: life on the edge of genomic stability. Curr. Opin. Oncol. 12, 74–81 (2000).
Hahn, W. C. et al. Inhibition of telomerase limits the growth of human cancer cells. Nature Med. 5, 1164–1170 (1999).
Zhang, X., Mar, V., Zhou, W., Harrington, L. & Robinson, M. O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13, 2388–2399 (1999).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 (1999).
Shresta, S., Pham, C. T., Thomas, D. A., Graubert, T. A. & Ley, T. J. How do cytotoxic lymphocytes kill their targets? Curr. Opin. Immunol. 10, 581–587 (1998).
Kagi, D. et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369, 31–37 (1994).
Heusel, J. W., Wesselschmidt, R. L., Shresta, S., Russell, J. H. & Ley, T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 76, 977–987 (1994).
Medema, J. P. et al. Cleavage of FLICE (caspase-8) by granzyme B during cytotoxic T lymphocyte-induced apoptosis. Eur. J. Immunol. 27, 3492–3498 (1997).
Heibein, J. A. et al. Granzyme B-mediated cytochrome c release is regulated by the Bcl-2 family members Bid and Bax. J. Exp. Med. 192, 1391–1402 (2000).
Rouvier, E., Luciani, M. F. & Golstein, P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J. Exp. Med. 177, 195–200 (1993).
Li, J. H. et al. The regulation of CD95 ligand expression and function in CTL. J. Immunol. 161, 3943–3949 (1998).
Herr, I. & Debatin, K. M. Cellular stress response and apoptosis in cancer therapy. Blood 98, 2603–2614 (2001).
Stahnke, K., Fulda, S., Friesen, C., Strauss, G. & Debatin, K. M. Activation of apoptosis pathways in peripheral blood lymphocytes by in vivo chemotherapy. Blood 98, 3066–3073 (2001).
Ryan, K. M., Phillips, A. C. & Vousden, K. H. Regulation and function of the p53 tumor suppressor protein. Curr. Opin. Cell Biol. 13, 332–337 (2001).
Sherr, C. J. The INK4A/ARF network in tumour suppression. Nature Rev. Mol. Cell Biol. 2, 731–737 (2001).
Miyashita, T. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Oda, E. et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288, 1053–1058 (2000).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Oda, K. et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102, 849–862 (2000).
Muller, M. et al. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 188, 2033–2045 (1998).
Guan, B., Yue, P., Clayman, G. L. & Sun, S. Y. Evidence that the death receptor DR4 is a DNA damage-inducible, p53-regulated gene. J. Cell Physiol. 188, 98–105 (2001).
Wu, G. S. et al. KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nature Genet. 17, 141–143 (1997).
Caelles, C., Helmberg, A. & Karin, M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370, 220–223 (1994).
Soengas, M. S. et al. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science 284, 156–159 (1999).
Bennett, M. et al. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science 282, 290–293 (1998).
Hug, H. et al. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J. Biol. Chem. 272, 28191–28193 (1997).
Friesen, C., Herr, I., Krammer, P. H. & Debatin, K. M. Involvement of the CD95 (APO-1/FAS) receptor/ligand system in drug- induced apoptosis in leukemia cells. Nature Med. 2, 574–577 (1996).Provides evidence that the CD95 system is involved in drug-induced apoptosis and so identifies one particular mechanism by which apoptosis is caused by anticancer drugs at the molecular level.
Fulda, S. et al. Activation of the CD95 (APO-1/Fas) pathway in drug- and γ-irradiation-induced apoptosis of brain tumor cells. Cell Death Differ. 5, 884–893 (1998).
Muller, M. et al. Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. J. Clin. Invest. 99, 403–413 (1997).
Muller, M., Scaffidi, C. A., Galle, P. R., Stremmel, W. & Krammer, P. H. The role of p53 and the CD95 (APO-1/Fas) death system in chemotherapy-induced apoptosis. Eur. Cytokine Netw. 9, 685–686 (1998).
Eichhorst, S. T. et al. A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol. Cell. Biol. 20, 7826–7837 (2000).
Newton, K. & Strasser, A. Ionizing radiation and chemotherapeutic drugs induce apoptosis in lymphocytes in the absence of Fas or FADD/MORT1 signaling. Implications for cancer therapy. J. Exp. Med. 191, 195–200 (2000).
Yeh, W. C. et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 279, 1954–1958 (1998).
Wesselborg, S., Engels, I. H., Rossmann, E., Los, M. & Schulze-Osthoff, K. Anticancer drugs induce caspase-8/FLICE activation and apoptosis in the absence of CD95 receptor/ligand interaction. Blood 93, 3053–3063 (1999).
Fulda, S. et al. Betulinic acid triggers CD95 (APO-1/Fas)- and p53-independent apoptosis via activation of caspases in neuroectodermal tumors. Cancer Res. 57, 4956–4964 (1997).
Tsujimoto, Y., Cossman, J., Jaffe, E. & Croce, C. M. Involvement of the BCL-2 gene in human follicular lymphoma. Science 228, 1440–1443 (1985).
McDonnell, T. J. et al. BCL-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell 57, 79–88 (1989).
Reed, J. C., Cuddy, M., Slabiak, T., Croce, C. M. & Nowell, P. C. Oncogenic potential of BCL-2 demonstrated by gene transfer. Nature 336, 259–261 (1988).
Strasser, A., Harris, A. W., Bath, M. L. & Cory, S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between MYC and BCL-2. Nature 348, 331–333 (1990).
Vaux, D. L., Cory, S. & Adams, J. M. BCL-2 gene promotes haemopoietic cell survival and cooperates with c-MYC to immortalize pre-B cells. Nature 335, 440–442 (1988).
Kogan, S. C. et al. BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor alpha chimeric protein (PMLRARα) to block neutrophil differentiation and initiate acute leukemia. J. Exp. Med. 193, 531–543 (2001).
Weller, M., Malipiero, U., Aguzzi, A., Reed, J. C. & Fontana, A. Protooncogene bcl-2 gene transfer abrogates Fas/APO-1 antibody-mediated apoptosis of human malignant glioma cells and confers resistance to chemotherapeutic drugs and therapeutic irradiation. J. Clin. Invest. 95, 2633–2643 (1995).
Campos, L. et al. High expression of BCL-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 81, 3091–3096 (1993).
Hermine, O. et al. Prognostic significance of BCL-2 protein expression in aggressive non- Hodgkin's lymphoma. Groupe d'Etude des Lymphomes de l'Adulte (GELA). Blood 87, 265–272 (1996).
Miyashita, T. & Reed, J. C. BCL-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 52, 5407–5411 (1992).
Schmitt, C. A., Rosenthal, C. T. & Lowe, S. W. Genetic analysis of chemoresistance in primary murine lymphomas. Nature Med. 6, 1029–1035 (2000).
Findley, H. W., Gu, L., Yeager, A. M. & Zhou, M. Expression and regulation of BCL-2, BCL-XL, and BAX correlate with p53 status and sensitivity to apoptosis in childhood acute lymphoblastic leukemia. Blood 89, 2986–2993 (1997).
Coustan-Smith, E. et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood 87, 1140–1146 (1996).
Henderson, S. et al. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl Acad. Sci. USA 90, 8479–8483 (1993).
Tarodi, B., Subramanian, T. & Chinnadurai, G. Epstein-Barr virus BHRF1 protein protects against cell death induced by DNA-damaging agents and heterologous viral infection. Virology 201, 404–407 (1994).
Sarid, R., Sato, T., Bohenzky, R. A., Russo, J. J. & Chang, Y. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nature Med. 3, 293–298 (1997).
Boise, L. H. et al. BCL-X, a BCL-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74, 597–608 (1993).
Dole, M. G. et al. BCL-XL is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res. 55, 2576–2582 (1995).
Nagane, M., Levitzki, A., Gazit, A., Cavenee, W. K. & Huang, H. J. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of BCL-XL and caspase-3-like proteases. Proc. Natl Acad. Sci. USA 95, 5724–5729 (1998).
Minn, A. J., Rudin, C. M., Boise, L. H. & Thompson, C. B. Expression of BCL-XL can confer a multidrug resistance phenotype. Blood 86, 1903–1910 (1995).
Zhou, P., Qian, L., Kozopas, K. M. & Craig, R. W. MCL-1, a BCL-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood 89, 630–643 (1997).
Kaufmann, S. H. et al. Elevated expression of the apoptotic regulator MCL-1 at the time of leukemic relapse. Blood 91, 991–1000 (1998).
Irmler, M. et al. Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 (1997).Describes the identification of the two forms of cellular FLIP and the expression of FLIP L in melanomas.
Mueller, C. M. & Scott, D. W. Distinct molecular mechanisms of Fas resistance in murine B lymphoma cells. J. Immunol. 165, 1854–1862 (2000).
Tepper, C. G. & Seldin, M. F. Modulation of caspase-8 and FLICE-inhibitory protein expression as a potential mechanism of Epstein–Barr virus tumorigenesis in Burkitt's lymphoma. Blood 94, 1727–1737 (1999).
Thome, M. et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386, 517–521 (1997).
Hu, S., Vincenz, C., Ni, J., Gentz, R. & Dixit, V. M. I-FLICE, a novel inhibitor of tumor necrosis factor receptor-1- and CD-95-induced apoptosis. J. Biol. Chem. 272, 17255–17257 (1997).
Bertin, J. et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl Acad. Sci. USA 94, 1172–1176 (1997).
Sturzl, M. et al. Expression of K13/v-FLIP gene of human herpesvirus 8 and apoptosis in Kaposi's sarcoma spindle cells. J. Natl Cancer Inst. 91, 1725–1733 (1999).
Kataoka, T. et al. FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and γ irradiation. J. Immunol. 161, 3936–3942 (1998).
Medema, J. P., de Jong, J., van Hall, T., Melief, C. J. & Offringa, R. Immune escape of tumors in vivo by expression of cellular FLICE- inhibitory protein. J. Exp. Med. 190, 1033–1038 (1999).Showed that expression of FLIP is a mechanism for immune escape of tumors in vivo.
Djerbi, M. et al. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190, 1025–1032 (1999).
Taylor, M. A. et al. Inhibition of the death receptor pathway by cFLIP confers partial engraftment of MHC class I-deficient stem cells and reduces tumor clearance in perforin-deficient mice. J. Immunol. 167, 4230–4237 (2001).
Cheng, J. et al. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263, 1759–1762 (1994).
Midis, G. P., Shen, Y. & Owen-Schaub, L. B. Elevated soluble Fas (sFas) levels in nonhematopoietic human malignancy. Cancer Res. 56, 3870–3874 (1996).
Ugurel, S., Rappl, G., Tilgen, W. & Reinhold, U. Increased soluble CD95 (sFas/CD95) serum level correlates with poor prognosis in melanoma patients. Clin. Cancer Res. 7, 1282–1286 (2001).
Gerharz, C. D. et al. Resistance to CD95 (APO-1/Fas)-mediated apoptosis in human renal cell carcinomas: an important factor for evasion from negative growth control. Lab. Invest. 79, 1521–1534 (1999).
Roth, W. et al. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 61, 2759–2765 (2001).
Reed, J. C. The Survivin saga goes in vivo. J. Clin. Invest. 108, 965–969 (2001).
Ambrosini, G., Adida, C. & Altieri, D. C. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med. 3, 917–921 (1997).Identifies the IAP-family member survivin and shows that some IAPs are overexpressed in cancers.
Adida, C., Berrebi, D., Peuchmaur, M., Reyes-Mugica, M. & Altieri, D. C. Anti-apoptosis gene, survivin, and prognosis of neuroblastoma. Lancet 351, 882–883 (1998).
Grossman, D. et al. Transgenic expression of survivin in keratinocytes counteracts UVB-induced apoptosis and cooperates with loss of p53. J. Clin. Invest. 108, 991–999 (2001).
Grossman, D., Kim, P. J., Schechner, J. S. & Altieri, D. C. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc. Natl Acad. Sci. USA 98, 635–640 (2001).
Mesri, M., Wall, N. R., Li, J., Kim, R. W. & Altieri, D. C. Cancer gene therapy using a survivin mutant adenovirus. J. Clin. Invest 108, 981–990 (2001).
Dierlamm, J. et al. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT, are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood 93, 3601–3609 (1999).
Vucic, D., Stennicke, H. R., Pisabarro, M. T., Salvesen, G. S. & Dixit, V. M. ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr. Biol. 10, 1359–1366 (2000).
Medema, J. P. et al. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc. Natl Acad. Sci. USA 98, 11515–11520 (2001).
Rampino, N. et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275, 967–969 (1997).
Meijerink, J. P. et al. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood 91, 2991–2997 (1998).
Molenaar, J. J. et al. Microsatellite instability and frameshift mutations in BAX and transforming growth factor-β RII genes are very uncommon in acute lymphoblastic leukemia in vivo but not in cell lines. Blood 92, 230–233 (1998).
Krajewski, S. et al. Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 55, 4471–4478 (1995).
Yin, C., Knudson, C. M., Korsmeyer, S. J. & Van Dyke, T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature 385, 637–640 (1997).
Bargou, R. C. et al. Overexpression of the death-promoting gene Bax-α which is downregulated in breast cancer restores sensitivity to different apoptotic stimuli and reduces tumor growth in SCID mice. J. Clin. Invest. 97, 2651–2659 (1996).
Ionov, Y., Yamamoto, H., Krajewski, S., Reed, J. C. & Perucho, M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc. Natl Acad. Sci. USA 97, 10872–10877 (2000).
Soengas, M. S. et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 409, 207–211 (2001).Showed that metastatic melanomas acquire resistance to chemotherapy by losing APAF1 expression.
Teitz, T. et al. Caspase-8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nature Med. 6, 529–535 (2000).
Strand, S. et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells — a mechanism of immune evasion? Nature Med. 2, 1361–1366 (1996).Showed that tumour cells can evade immune attack by downregulation of the CD95 receptor and killing of lymphocytes through expression of CD95L.
Moller, P. et al. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int. J. Cancer 57, 371–377 (1994).
Leithauser, F. et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab. Invest. 69, 415–429 (1993).
Volkmann, M. et al. Loss of CD95 expression is linked to most but not all p53 mutants in European hepatocellular carcinoma. J. Mol. Med. 79, 594–600 (2001).
Peli, J. et al. Oncogenic Ras inhibits Fas ligand-mediated apoptosis by downregulating the expression of Fas. EMBO J. 18, 1824–1831 (1999).
Maeda, T. et al. Fas gene mutation in the progression of adult T cell leukemia. J. Exp. Med. 189, 1063–1071 (1999).
Landowski, T. H., Qu, N., Buyuksal, I., Painter, J. S. & Dalton, W. S. Mutations in the Fas antigen in patients with multiple myeloma. Blood 90, 4266–4270 (1997).
Cascino, I., Papoff, G., De Maria, R., Testi, R. & Ruberti, G. Fas/Apo-1 (CD95) receptor lacking the intracytoplasmic signaling domain protects tumor cells from Fas-mediated apoptosis. J. Immunol. 156, 13–17 (1996).
Straus, S. E. et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood 98, 194–200 (2001).
Shin, M. S. et al. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 61, 4942–4946 (2001).
Fisher, M. J. et al. Nucleotide substitution in the ectodomain of trail receptor DR4 is associated with lung cancer and head and neck cancer. Clin. Cancer Res. 7, 1688–1697 (2001).
Pai, S. I. et al. Rare loss-of-function mutation of a death receptor gene in head and neck cancer. Cancer Res 58, 3513–3518 (1998).
Lee, S. H. et al. Alterations of the DR5/TRAIL receptor 2 gene in non-small cell lung cancers. Cancer Res. 59, 5683–5686 (1999).
Liston, P. et al. Identification of XAF1 as an antagonist of XIAP anti-caspase activity. Nature Cell Biol. 3, 128–133 (2001).
Lowe, S. W. et al. p53 status and the efficacy of cancer therapy in vivo. Science 266, 807–810 (1994).Showed that tumours can acquire resistance to therapy by mutation or deficiency of p53.
Lee, J. M. & Bernstein, A. p53 mutations increase resistance to ionizing radiation. Proc. Natl Acad. Sci. USA 90, 5742–5746 (1993).
Aas, T. et al. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nature Med. 2, 811–814 (1996).
Bunz, F. et al. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J. Clin. Invest. 104, 263–269 (1999).
Schmitt, C. A., McCurrach, M. E., de Stanchina, E., Wallace-Brodeur, R. R. & Lowe, S. W. INK4A/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 13, 2670–2677 (1999).
Samuels-Lev, Y. et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol. Cell 8, 781–794 (2001).
Kauffmann-Zeh, A. et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385, 544–548 (1997).
Chang, H. W. et al. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 276, 1848–1850 (1997).
Shayesteh, L. et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nature Genet. 21, 99–102 (1999).
Podsypanina, K. et al. Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc. Natl Acad. Sci. USA 96, 1563–1568 (1999).
Suzuki, A. et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8, 1169–1178 (1998).
Steck, P. A. et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23. 3 that is mutated in multiple advanced cancers. Nature Genet. 15, 356–362 (1997).
Cheng, J. Q. et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl Acad. Sci. USA 89, 9267–9271 (1992).
Persidis, A. Cancer multidrug resistance. Nature Biotechnol. 17, 94–95 (1999).
Cole, S. P. et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258, 1650–1654 (1992).
Johnstone, R. W., Cretney, E. & Smyth, M. J. P-glycoprotein protects leukemia cells against caspase-dependent, but not caspase-independent, cell death. Blood 93, 1075–1085 (1999).
Smyth, M. J., Krasovskis, E., Sutton, V. R. & Johnstone, R. W. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc. Natl Acad. Sci. USA 95, 7024–7029 (1998).
Bours, V. et al. Nuclear factor-κB, cancer, and apoptosis. Biochem. Pharmacol. 60, 1085–1089 (2000).
Rayet, B. & Gelinas, C. Aberrant REL/NF-κB genes and activity in human cancer. Oncogene 18, 6938–6947 (1999).
Wood, K. M., Roff, M. & Hay, R. T. Defective IκBα in Hodgkin cell lines with constitutively active NF-κB. Oncogene 16, 2131–2139 (1998).
Sethi, T. et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nature Med. 5, 662–668 (1999).
Catlett-Falcone, R. et al. Constitutive activation of STAT3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10, 105–115 (1999).
Nicholson, D. W. From bench to clinic with apoptosis-based therapeutic agents. Nature 407, 810–816 (2000).
Walczak, H. et al. Tumoricidal activity of tumor necrosis factor-related apoptosis- inducing ligand in vivo. Nature Med. 5, 157–163 (1999).Showed that a recombinant form of TRAIL (LZ-TRAIL) could suppress tumour growth in vivo without affecting normal tissue.
Keane, M. M., Ettenberg, S. A., Nau, M. M., Russell, E. K. & Lipkowitz, S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 59, 734–741 (1999).
Chinnaiyan, A. M. et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc. Natl Acad. Sci. USA 97, 1754–1759 (2000).
Nagane, M. et al. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 60, 847–853 (2000).
Fransen, L., Van der Heyden, J., Ruysschaert, R. & Fiers, W. Recombinant tumor necrosis factor: its effect and its synergism with interferon-γ on a variety of normal and transformed human cell lines. Eur. J. Cancer Clin. Oncol. 22, 419–426 (1986).
Lienard, D., Ewalenko, P., Delmotte, J. J., Renard, N. & Lejeune, F. J. High-dose recombinant tumor necrosis factor alpha in combination with interferon γ and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J. Clin. Oncol. 10, 52–60 (1992).
Daniel, P. T., Wieder, T., Sturm, I. & Schulze-Osthoff, K. The kiss of death: promises and failures of death receptors and ligands in cancer therapy. Leukemia 15, 1022–1032 (2001).
Ellerby, H. M. et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nature Med. 5, 1032–1038 (1999).
Jansen, B. et al. BCL-2 antisense therapy chemosensitizes human melanoma in SCID mice. Nature Med. 4, 232–234 (1998).
Waters, J. S. et al. Phase I clinical and pharmacokinetic study of BCL-2 antisense oligonucleotide therapy in patients with non-Hodgkin's lymphoma. J. Clin. Oncol. 18, 1812–1823 (2000).
Taylor, J. K., Zhang, Q. Q., Wyatt, J. R. & Dean, N. M. Induction of endogenous BCL-xS through the control of Bcl-X pre-mRNA splicing by antisense oligonucleotides. Nature Biotechnol. 17, 1097–1100 (1999).
Zamore, P. D. RNA interference: listening to the sound of silence. Nature Struct. Biol. 8, 746–750 (2001).
Bullock, A. N. & Fersht, A. R. Rescuing the function of mutant p53. Nature Rev. Cancer 1, 68–76 (2001).
Yang, C., Cirielli, C., Capogrossi, M. C. & Passaniti, A. Adenovirus-mediated wild-type p53 expression induces apoptosis and suppresses tumorigenesis of prostatic tumor cells. Cancer Res. 55, 4210–4213 (1995).
Asgari, K. et al. Inhibition of the growth of pre-established subcutaneous tumor nodules of human prostate cancer cells by single injection of the recombinant adenovirus p53 expression vector. Int. J. Cancer 71, 377–382 (1997).
Wang, C. Y., Cusack, J. C. Jr, Liu, R. & Baldwin, A. S. Jr. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nature Med. 5, 412–417 (1999).
Huang, D. C. & Strasser, A. BH3-only proteins-essential initiators of apoptotic cell death. Cell 103, 839–842 (2000).
Datta, S. R. et al. AKT phosphorylation of BAD couples survival signals to the cell- intrinsic death machinery. Cell 91, 231–241 (1997).
Vogt, C. Untersuchungen über die Entwicklungsgeschichte der Geburtshelferkroete (Alytes obstetricians) (Jent & Gassmann, Solothurn, 1842).
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the European Community, the Deutsche Krebshilfe, the Sander Stiftung, the Tumor Center Heidelberg/Mannheim, the BMBF and the German-Israeli Cooperation in Cancer Research. We thank A. Krueger for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
GenBank
LocusLink
OMIM
autoimmune lymphoproliferative syndrome (ALPS)
Medscape DrugInfo
FURTHER INFORMATION
Glossary
- LAMINS
-
A group of intermediate-filament proteins that form the fibrous network (nuclear lamina) on the inner surface of the nuclear envelope.
- INTEGRINS
-
A large family of heterodimeric transmembrane proteins that promote adhesion of cells to the extracellular matrix or to other cells.
- ADAPTIVE IMMUNE SYSTEM
-
Adaptive immunity — also known as specific or acquired immunity — is mediated by antigen-specific lymphocytes and antibodies; it is highly antigen specific and includes the development of immunological memory.
- INNATE IMMUNE SYSTEM
-
The innate immune system includes phagocytes, natural killer cells, the complement system and other non-specific components. It protects against infections using mechanisms that exist before infection, providing a rapid response to microbes that is essentially the same regardless of the type of infection.
- ANTIMETABOLITES
-
Antimetabolites (for example, methotrexate) block specific metabolic pathways by competitive binding to the substrate-binding site of enzymes that are involved in metabolism.
- TOPOISOMERASES
-
A class of enzymes that control the number and topology of supercoils in DNA and that are important for DNA replication.
- SUMOYLATION
-
A post-translational modification that consists of covalent attachment of the small ubiquitin-like molecule, SUMO-1 (also known as sentrin, PIC1). Sumoylation can change the ability of the modified protein to interact with other proteins and can interfere with its proteasomal degradation.
- DOXORUBICIN
-
A chemotherapeutic drug that induces DNA strand breaks, which initiate apoptosis.
- STAT3
-
A member of the STAT (signal transducer and activator of transcription) family of transcription factors. STATs are activated through phosphorylation by Janus kinases and have an important role in cytokine receptor signalling.
- RNA INTERFERENCE
-
(RNAi). Use of double-stranded RNA to target specific mRNAs for degradation, resulting in sequence-specific post-transcriptional gene silencing.
Rights and permissions
About this article
Cite this article
Igney, F., Krammer, P. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer 2, 277–288 (2002). https://doi.org/10.1038/nrc776
Issue Date:
DOI: https://doi.org/10.1038/nrc776
This article is cited by
-
CDK5 promotes apoptosis and attenuates chemoresistance in gastric cancer via E2F1 signaling
Cancer Cell International (2023)
-
Inhibition of DAMP actions in the tumoral microenvironment using lactoferrin-glycyrrhizin conjugate for glioblastoma therapy
Biomaterials Research (2023)
-
Apoptotic bodies: bioactive treasure left behind by the dying cells with robust diagnostic and therapeutic application potentials
Journal of Nanobiotechnology (2023)
-
Antiproliferative and apoptotic effects of telmisartan in human glioma cells
Cancer Cell International (2023)
-
Laser-activatable oxygen self-supplying nanoplatform for efficiently overcoming colorectal cancer resistance by enhanced ferroptosis and alleviated hypoxic microenvironment
Biomaterials Research (2023)