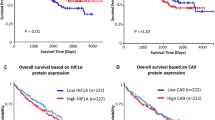
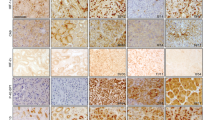
Abstract
The VHL gene product (pVHL) forms a multimeric complex with the elongin B and C, Cul2 and Rbx1 proteins (VCBCR complex), which is homologous to the SCF family of ubiquitin ligase complexes. The VCBCR complex binds HIF-1α and HIF-2α, transcription factors critically involved in cellular responses to hypoxia, and targets them for ubiquitin-mediated proteolysis. Germline mutations in the VHL gene cause susceptibility to haemangioblastomas, renal cell carcinoma (RCC), phaeochromocytoma and other tumours. In addition somatic inactivation of the VHL gene occurs in most sporadic clear cell RCC (CC-RCC). However, the absence of somatic VHL inactivation in 30–40% of CC-RCC implies the involvement of other gatekeeper genes in CC-RCC development. We reasoned that in CC-RCC without VHL inactivation, other pVHL-interacting proteins might be defective. To assess the role of elongin B/C, Rbx1 and HIF-1α in RCC tumorigenesis we (a) mapped the genes to chromosomes 8q(cen) (elongin C), 16p13.3 (elongin B) and 22q11.2 (Rbx1) by FISH, monochromosomal somatic cell hybrid panel screening and in silico GenBank homology searching; (b) determined the genomic organisation of elongin C (by direct sequencing of PAC clones), Rbx1 and elongin B (by GenBank homology searching); and (c) performed mutation analysis of exons comprising the coding regions of elongins B, C and Rbx1 and the oxygen-dependent degradation domain of HIF-1α by SSCP screening and direct sequencing in 35 sporadic clear cell RCC samples without VHL gene inactivation and in 13 individuals with familial non-VHL clear cell RCC. No coding region sequence variations were detected for the elongin B, elongin C or Rbx1 genes. Two amino acid substitutions (Pro582Ser and Ala588Thr) were identified in the oxygen-dependent degradation/pVHL binding domain of HIF-1α, however neither substitution was observed exclusively in tumour samples. Association analysis in panels of CC-RCC and non-neoplastic samples using the RFLPs generated by each variant did not reveal allelic frequency differences between RCC patients and controls (P>0.32 by chi-squared analysis). Nevertheless, the significance of these variations and their potential for modulation of HIF-1α function merits further investigation in both other tumour types and in non-neoplastic disease. Taken together with our previous Cul2 mutation analysis these data suggest that development of sporadic and familial RCC is not commonly contributed to by genetic events altering the destruction domain of HIF-1α, or components of the HIF-α destruction complex other than VHL itself. Although (a) activation of HIF could occur through mutation of another region of HIF-a, and (b) epigenetic silencing of elongin B/C, Cul2 or Rbx1 cannot be excluded, these findings suggest that pVHL may represent the sole mutational target through which the VCBR complex is disrupted in CC-RCC. HIF response is activated in CC-RCC tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER . 1998 Genes Chrom. Cancer 22: 200–209
Clifford SC, Walsh S, Hewson K, Green EK, Brinke A, Green PM, Gianelli F, Eng C, Maher ER . 1999 Genes Chrom. Cancer 26: 20–28
Cockman ME, Masson N, Mole DR, Jaakkola P, Chang GW, Clifford SC, Maher ER, Pugh CW, Ratcliffe PJ, Maxwell PH . 2000 J. Biol. Chem. 275: 25733–25741
Crossey PA, Richards FM, Foster K, Green JS, Prowse A, Latif F, Lerman MI, Zbar B, Affara NA, Ferguson-Smith MA et al . 1994 Hum. Mol. Genet. 3: 1303–1308
Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP . 2000 Nat. Genet. 25: 315–319
De Sepulveda P, Ilangumaran S, Rottapel R . 2000 J. Biol. Chem. 275: 14005–14008
Duerr EM, Gimm O, Neuberg DS, Kum JB, Clifford SC, Toledo SP, Maher ER, Dahia PL, Eng C . 1999 J. Clin. Endocrinol. Metab. 84: 3207–3211
Fearon ER . 1997 Science 278: 1043–1050
Foster K, Crossey PA, Cairns P, Hetherington JW, Richards FM, Jones MH, Bentley E, Affara NA, Ferguson-Smith MA, Maher ER . 1994 Br. J. Cancer 69: 230–234
Gailani MR, Stahlebackdahl M, Leffell DJ, Glynn M, Zaphiropoulos PG, Pressman C, Unden AB, Dean M, Brash DE, Bale AE, Toftgard R . 1996 Nat. Genet. 14: 78–81
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM et al . 1994 Nat. Genet. 7: 85–90
Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM et al . 1994 Proc. Natl. Acad. Sci. USA 91: 9700–9704
Huang LE, Gu J, Schau M, Bunn HF . 1998 Proc. Natl. Acad. Sci. USA 95: 7987–7992
Ioannou PA, de Jong PJ . 1996 Construction of bacterial artificial chromosome libraries using the modified P1 (PAC) system In Dracopoli et al. (ed.) Current Protocols in Human Genetics Unit 5.15 New York: John Wiley and Sons
Iyer NV, Leung SW, Semenza GL . 1998 Genomics 52: 159–165
Kadota M, Tamaki Y, Sakita I, Komoike Y, Miyazaki M, Ooka M, Masuda N, Fujiwara Y, Ohnishi T, Tomita N, Sekimoto M, Ohue M, Ikeda T, Kobayashi T, Horii A, Monden M . 2000 Onco. Rep. 7: 529–533
Kaelin Jr WG, Maher ER . 1998 Trends Genet. 14: 423–426
Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW . 2000 Proc. Natl. Acad. Sci. USA 97: 10430–10435
Kelsell DP, Rooke L, Warne D, Bouzyk M, Cullin L, Cox S, West L, Povey S, Spurr NK . 1995 Ann. Hum. Genet. 59: 233–241
Knowles MA, Shaw ME, Proctor AJ . 1993 Oncogene 8: 1357–1364
Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H . 1997 Science 275: 1784–1787
Lininger RA, Park WS, Man YG, Pham T, MacGrogan G, Zhuang Z, Tavassoli FA . 1998 Hum. Pathol. 29: 1113–1118
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ . 1999 Nature 399: 271–275
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW . 1997 Science 275: 1787–1790
Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG . 2000 Nat. Cell. Biol. 2: 423–427
Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T . 1989 Proc. Natl. Acad. Sci. USA 86: 2766–2770
Perinchery G, Bukurov N, Nakajima K, Chang J, Hooda M, Oh BR, Dahiya R . 1999 Int. J. Oncol. 14: 495–500
Poli-Frederico RC, Bergamo NA, Reis PP, Kowalski LP, Zielenska M, Squire JA, Rogatto SR . 2000 Head Neck 22: 585–590
Pugh CW, O'Rourke JF, Nagao M, Gleadle JM, Ratcliffe PJ . 1997 J. Biol. Chem. 272: 11205–11214
Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y . 2000 Nat. Genet. 24: 245–250
Schofield DE, Beckwith JB, Sklar J . 1996 Genes Chrom. Cancer 15: 10–17
Semenza GL, Rue EA, Iyer NV, Pang MG, Kearns WG . 1996 Genomics 34: 437–439
Semenza GL . 2000 Genes Dev. 14: 1983–1991
Shapiro MB, Senapathy P . 1987 Nuc. Acids Res. 15: 7155–7175
Sparks AB, Morin PJ, Vogelstein B, Kinzler KW . 1998 Cancer Res. 58: 1130–1134
Tanimoto K, Makino Y, Pereira T, Poellinger L . 2000 EMBO J. 19: 4298–4309
The BT, Giraud S, Sari NF, Hii SI, Bergerat JP, Larsson C, Limacher JM, Nicol D . 1997 Lancet 349: 848–849
Thoenes W, Storkel S, Rumpelt HJ . 1986 Path. Res. Prac. 181: 125–143
Wolter M, Reifenberger J, Sommer C, Ruzicka T, Reifenberger G . 1997 Cancer Res. 57: 2581–2585
Woodward ER, Clifford SC, Astuti D, Affara NA, Maher ER . 2000 J. Med. Genet. 37: 348–353
Xie JW, Murone M, Luoh SM, Ryan A, Gu QM, Zhang CH, Bonifas JM, Lam CW, Hynes M, Goddard A, Rosenthal A, Epstein EH, de Sauvage FJ . 1998 Nature 391: 90–92
Acknowledgements
We thank the Medical Research Council, Cancer Research Campaign (CRC) and the VHL Alliance for financial support. We are grateful to Dr PC Harris, Mayo Clinic for providing DNA samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clifford, S., Astuti, D., Hooper, L. et al. The pVHL-associated SCF ubiquitin ligase complex: Molecular genetic analysis of elongin B and C, Rbx1 and HIF-1α in renal cell carcinoma. Oncogene 20, 5067–5074 (2001). https://doi.org/10.1038/sj.onc.1204602
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1204602
Keywords
This article is cited by
-
Mitochondrial MUL1 E3 ubiquitin ligase regulates Hypoxia Inducible Factor (HIF-1α) and metabolic reprogramming by modulating the UBXN7 cofactor protein
Scientific Reports (2020)
-
Association of hypoxia-inducible factor-1α (HIF1α) 1790G/A gene polymorphism with renal cell carcinoma and prostate cancer susceptibility: a meta-analysis
BMC Medical Genetics (2019)
-
ARD1/NAA10 in hepatocellular carcinoma: pathways and clinical implications
Experimental & Molecular Medicine (2018)
-
A meta-analysis of hypoxia inducible factor 1-alpha (HIF1A) gene polymorphisms: association with cancers
Biomarker Research (2015)
-
HIF-1α (1772C>T) polymorphism as marker for breast cancer development
Tumor Biology (2015)