Article Text
Abstract
AIMS To determine the incidence of neonatal thromboembolism in Germany.
METHODS Diagnostic imaging techniques, therapeutic modalities, and short term outcome were evaluated in a prospective nationwide two year case registry study.
RESULTS The reported incidence of symptomatic neonatal thromboembolism, diagnosed in most cases with Doppler ultrasonography, was 5.1 per 100 000 births, with a total of 79 cases registered: renal venous thrombosis (n=35); venous thrombosis (n=25); and arterial vascular occlusion (n=19). Fifty seven of 79 thromboses were associated with additional risk factors (central line n=25, asphyxia n=13, septicaemia n=11, dehydration n=6, maternal diabetes n=2, cardiac disease n=1). Inherited thrombophilia was also diagnosed in seven out of 35 cases investigated. Twenty three children received supportive treatment: 42 received heparin and in 13 neonates thrombolytic agents were administered. Most neonates (91%) survived; seven died.
CONCLUSION Controlled multicentre studies are needed to obtain more information on treatment efficacy.
- thromboembolism
- Doppler ultrasonography
- heparin
- thrombolytic agents
- Germany
Statistics from Altmetric.com
The established incidence of hereditary thrombophilia is 1 in 2500 to 1 in 5000.1 However, the manifestation of inherited thrombotic disorders occurs in less than 5% of affected children compared with about 40% of adults.2 Most infants and children with thromboembolytic diseases have several associated risk factors before their vascular occlusion occurs, such as peripartum asphyxia, fetal diabetes, dehydration, septicaemia, malignant disease and/or anti-neoplastic polychemotherapy, central lines, trauma, surgery or raised antiphospholipid antibody titres.2-6 Neonates are at the greatest risk of childhood thromboembolytic complications and the incidence decreases significantly after the first year of life. Although relatively rarely reported, neonatal thromboembolism may result in death or severe organ failure as a result of irreversible tissue damage.7-11 The incidence of neonatal thromboembolism, currently performed diagnostic imaging techniques, therapeutic modalities and short term outcome were evaluated in a prospective two year survey performed between July 1992 and June 1994.
Methods
At monthly intervals 450 heads of paediatric healthcare centres in Germany, including neonatal and haematology wards, received an inquiry concerning the occurrence of symptomatic neonatal thrombosis. When neonatal thromboembolism was reported, they were asked to complete a detailed five page questionnaire to provide information on the demographic details of their patients, the specific site of vascular occlusion, additional risk factors, familial thrombophilia, diagnostic imaging techniques currently in use, therapeutic modalities, vascular outcome and survival rate.
Laboratory tests performed in the collaborating hospitals included the following assays: platelet count, activated partial thromboplastin time, protein C, protein S, antithrombin and factor XII. The results were compared with those for normal age related values.12 13 Furthermore, in neonates with suspected inherited thrombophilia at the time when thromboembolism occurred, the tests were repeated when they had completed their course of treatment. The final diagnosis was made when familial screening confirmed the suspected inherited coagulation defect.
Median and range calculations and statistics (Wilcoxon-Mann-Whitney U tests; χ2 test) were performed using the Stat view version 4.02 program.
Results
The total number of registered cases during the period under study was 103. However, after reviewing the submitted questionnaires we excluded 24 patients in whom thromboembolism occurred before July 1992 (n=10) or after the neonatal period (> 4 weeks of age; n= 3). A further 11 cases in whom the diagnosis had not been confirmed radiologically (n=5) and cases of questionable data (n=6) were excluded.
Using data from the corresponding population based denominator, the National Birth Register, the incidence of the remaining 79 thrombotic events in the neonatal period was calculated to be 5.1 per 100 000 births.
DISTRIBUTION OF NEONATAL THROMBOEMBOLISM
Demographic characteristics are shown in table 1. Reported cases were assigned to one of three groups according to the site of thromboembolism: renal venous thrombosis (RVT: n=35 or 44%) venous thrombosis located at other sites (VT: n=25 or 32%), and arterial vascular insults (AT: n=19 or 24%). No significant differences for site of the thrombus were recorded for gestational age or birthweight in term or preterm babies, respectively. In contrast, we found a male predominance of renal venous thrombosis in term infants. Figure 1 shows the different age distributions at diagnosis of vascular insults for term and preterm infants: whereas RVT occurred at a median of eight days in preterm infants, term neonates developed RVT, the most commonly recorded vascular occlusion in this group, significantly (U-test: P = 0.002) sooner after birth. Arterial insults followed a similar pattern to that of RVT and occurred significantly (U-test: P = 0.009) earlier at day 1 (median) in term infants. In preterm babies arterial vascular occlusion was diagnosed at the end of the first week of life. In contrast, VT showed no such significant difference in incidence; in both groups vascular insults were diagnosed in a median of 11 or 12 days, respectively.
Demographic patient characteristics in neonatal thromboembolism
Different age distribution at time of diagnosis in renal venous thrombosis (RVT), venous thrombosis (VT), and arterial vascular occlusion (AT) in preterm or term neonates (median and SE values). Compared with preterm babies, term infants developed RVT (P= 0.002) or AT significantly earlier (P=0.009).
THROMBUS LOCATION
VT involved the superior caval vein (n=6), the femoral vein (n=5), the inferior caval vein (n=4), the portal vein (n=2), the right atrium (n=3), the axillary vein (n=1), and retinal vascular occlusion (n=1). One of the 25 neonates with venous thrombosis also had an arterial embolism. Multiple VT was diagnosed in three of the 25 neonates. Arterial vascular insults affected the femoral or iliac arteries (n=8), the cerebral arteries (n=7), the aorta (n=2), the umbilical artery (n=1) and the left atrium (n=1). In contrast, symptomatic central venous thrombosis was not recorded in the period studied.
IMAGING
When symptomatic neonatal thromboembolism was clinically suspected, Doppler ultrasonography was performed in most cases to document thrombosis. Less commonly used were contrast angiography or magnetic resonance angiography (10 out of 79 cases), which were performed mainly when the central nervous system was involved.
RISK FACTORS
Seventy per cent (n=57) of all thrombotic events (n=79) were associated with additional risk factors precipitating the vascular insult: 25 out of 57 thromboses were associated with indwelling central lines. Systemic septicaemia (n=11) or asphyxia (n=13) were the next most common risk factors. Dehydration (n=6) and congenital heart disease (n=1) were also documented. Maternal diabetes leading to neonatal vascular occlusion was diagnosed in one case of arterial and in one of venous thrombosis. Central lines were commonly associated with venous thrombosis (preterm n=10; term n=7), in most of the cases diagnosed in the second week of life in the upper venous system, followed by RVT or AT in four cases (χ2 12.125; P = 0.0023). The remaining 22 neonates had spontaneous thrombosis without additional risk factors. In children with arterial occlusion there was no difference in the distribution of the risk factors mentioned.
GENETIC RISK FACTORS FROM FAMILIAL THROMBOPHILIA
In 35 of the 79 children inherited coagulation disorders were investigated: heterozygous protein C type I deficiency was diagnosed in five cases; protein S and factor XII deficiencies were repeatedly found in two patients, respectively. No antithrombin deficiency was reported. Interestingly, familial thrombophilia was associated only twice with peripartal asphyxia.
TREATMENT MODALITIES
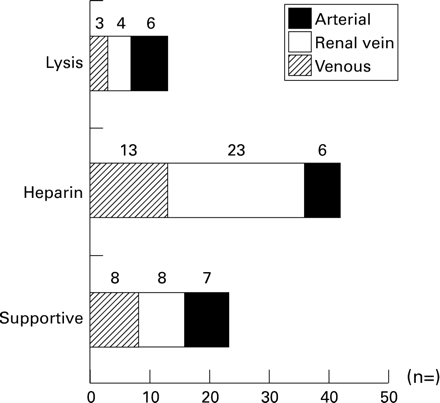
Due to local treatment preferences, the treatment of neonatal thrombosis varied greatly (fig 2). Supportive care was performed in 23 cases. Full anticoagulant treatment with heparin in a median (range) dose of 300 (250-1000) IU/kg/day was administered to most of the neonates (RVT n=23, VT n=13, AT n=6). In 13 of the 79 cases initial thrombolytic treatment was performed with streptokinase (n=3), urokinase (n=2) or recombinant tissue plasminogen activator (n=8). Due to non-response within the first three days of treatment a cross-over to urokinase (t-PA n=1) or t-PA (streptokinase n=1; urokinase n=1) was reported.
Treatment modalities of neonatal thromboembolism for arterial and venous sites.
Tables 2 and 3 show the location of vascular occlusion, estimated age of thrombosis, thrombolytic agents and doses administered. One neonate in the heparin group was reported to be having increasing intraventricular bleeding during heparin administration, but mucosal bleeding (n=1) or bleeding from puncture sites (n=1) also occurred during rt-PA thrombolysis. Systemic hyperfibrinolysis was observed in a neonate during urokinase infusion.
Patient characteristics (thrombolytic treatment)
Patient characteristics (thrombolytic treatment)
SHORT TERM OUTCOME
Figure 3 shows the outcome related to the site of thromboembolism: complete resolution was recorded in 26 cases, partial patency was achieved in 21 patients, and no changes were observed in 25 children with RVT, VT, or AT, respectively.
Patency of neonatal thromboembolism according to arterial and venous sites.
Figure 4 shows short term outcome for each of the treatments: whereas supportive care was described as failing to achieve patency in most of the cases reported, there was no difference in those children given heparin. In contrast, in the small thrombolytic group nine out of 13 patients achieved complete patency. The different outcome reported for the treatment modalities administered was significant (χ29.522; P = 0.049).
Patency of neonatal thromboembolism according to different treatment modalities.
Seven of the 79 infants with thromboses died: RVT (n=1),VT (n=3), and AT (n=3). Death occurred in term (n=1) or premature babies due to underlying disease (n=3) or multiple cerebral artery occlusion (n=2), or the cause was diagnosed at necropsy (superior caval vein with involvement of the right atrium: (n=1).
Discussion
As far as we are aware there has only been one major prospective study on thromboembolism in the neonatal period. It reported 2.4 clinically apparent thromboses per 1000 admissions to neonatal intensive care units.5 However, because the demographics and population density in Canada differ from those of Germany, we obtained representative data in a prospective two year survey performed between July 1992 and June 1994. This nationwide prospective questionnaire suggests the incidence to be 5.1 symptomatic thromboses per 100 000 births, diagnosed and treated not only at intensive care units but also on neonatal wards. The data from this survey also provide more information on neonates presenting with thromboembolism, additional risk factors, currently performed diagnostic imaging techniques, different therapeutic modalities and short term outcome.
Although the return of the monthly inquiry averaged 80–90% for the period studied, incomplete reporting cannot definitely be excluded. However, because this study focused on symptomatic neonatal thrombosis under reporting is likely as some cases might not have come to the attention of the reporting physician.
Besides RVT, most venous thromboses diagnosed in the second week of life in premature or mature infants were located in the upper venous system and associated with indwelling central lines. In this survey about a third (RVT) and half (VT or AT), respectively, of vascular insults were reported in premature babies treated at intensive care units. Compared with term infants, preterm babies had symptoms of RVT or AT significantly later at the end of the first week of life, and these were more often associated with central lines. These data therefore provide evidence that preterm infants are at a higher risk of developing vascular occlusions than mature infants. Furthermore, especially in the neonatal period, the slow double capillary circulation in the kidney is probably particularly vulnerable to thrombosis if blood concentration, dehydration, or hypercoagulation occur.
In contrast to the Canadian thrombosis registry,5 half the reported arterial vascular insults diagnosed in the first week of life affected cerebral arteries; possible thromboembolism due to further thrombosis in the venous system was excluded in most of the cases reported. Besides neonatal stroke the femoral and iliac arteries were affected with the same frequency. Only two cases of aortal thrombosis were reported.
Inherited coagulation defects predisposing to vascular occlusion were found in seven out of 35 neonates investigated. Due to the difficulties of drawing enough blood for coagulation studies in sick premature or mature infants with serious illness treated at intensive care units, one of the limitations of the published reports,4 5 and of this questionnaire, is that in only a small number of infants were genetic defects of familial thrombophilia investigated. As the study was carried out between 1992 and 1994, there was no laboratory screening for resistance to activated protein C at this time. Evidence suggests that resistance to activated protein C and further defects of the protein C pathway14 may have a major role in neonatal stroke15 or in neonates with thromboembolism after cardiac catheterisation.6 Preliminary data from a prospective study in neonates and children with central lines show that genetic defects of thrombophilia have a major role in catheter related thrombosis: in a population of 163 children with central lines flushed with low dose heparin (0.15-0.3 IU/ml anti-Xa activity), 15 out of 18 children with familial thrombophilia developed vascular occlusion (Nowak-Göttl et al, unpublished observations). In contrast, as previously reported by Vielhaber et al,6 two children with familial thrombophilia and heparin adjusted to 0.6 anti-Xa activity did not develop thrombosis. Additional risk factors for vascular occlusion, such as central lines, may therefore be more important and promote the early manifestation of thromboembolism in neonates with genetic defects of familial thrombophilia.6 15
Although the recommended gold standard imaging technique for thrombosis is angiography or phlebography,16 in most cases, Doppler ultrasonography, which can easily be performed in intensive care units, was used to document neonatal vascular insults apart from thromboembolism located in the central nervous system. As no prospective randomised data on different imaging techniques are available in this age group, this non-invasive method is useful when carried out by experienced clinicians.
Treatment of neonatal vascular insults reported in this questionnaire varied greatly and included supportive care and anticoagulation with heparin in most of the children affected. However, although we found the statistically best results in patency in the thrombolitic group and the best short term outcome in patients treated with supportive care, no conclusions can be drawn from this study about the long term efficacy of the different treatment modalities applied. Controlled, randomised, multicentre studies are needed to obtain more information on this, especially the use of heparin compared with thrombolitic agents.
Acknowledgments
We gratefully acknowledge the support given by the following colleagues: H Hörnchen (Aachen), D M Gabriel (Aschaffenburg), R G Schmid (Altötting), H Versmold, E L Grauel (Berlin), H W Schenk (Böblingen), S Kowalewski (Bonn), E Krüger (Brandenburg), H Bachmann (Bremen), H Jacobi (Celle), U Schamberger (Coburg), H Isenberg (Darmstadt), L Diekmann (Dortmund), D Gmyrek (Dresden), K J Eßler (Düren-Biresdorf), B Adrian (Eberswalde-Finow), W Havers, U Stephan (Essen), R Schlößer (Frankfurt), M Brandis (Freiburg), C Baisch (Friedrichshafen), F de Souza (Gifthorn), D M Eder (Halberstadt), E Kattner (Hamburg), E Fukala (Halle), C Wieg (Hanau), A Bökenkamp, J Natzschka (Hanover), R Muchow (Herford), J Kerstan (Hildesheim), H G Limbach (Homburg/Saar), F Schindera (Karlsruhe), H Wehinger (Kassel), H Schuhmacher (Kleve), U Knop (Köln), R Herterich (Landshut), M Stahl (Lörrach), K Kruse (Lübeck), H P Weber (Lüdenscheid), D Schulz (Lüneburg), L Ritter (Magdeburg), J Spranger (Mainz), R Burghard (Memmingen), K Auberger, P Emmrich, W Lindner, G Haus, R Roos, K D Tymner (Munich), I A Henrichs (Neuburg), U Keuth (Neukirchen/Saar), C Bistrup (Neuss), F J Helmig (Ravensburg), C Schütz (Regensburg), F K Trefz (Reutlingen), C Plath (Rostock), H Günther (Saarlouis), H Giesen (Schweinfurt), M Thiemeyer (Soest), V Siller (Stollberg), H Rebmann (Tübingen), T Luthardt (Worms), K Runge (Wuppertal).
We also thank S Griesbach for helpful comments and B Heinrichs for administrative help.